Submitting Establishment Information to the Animal Drug Manufacturing System (ADMS)
Content
Q1. What primary submission types may require ADMS Information?
Q2. What types of submission reactivations may require ADMS information?
Q3. What primary submission types may not require ADMS Information?
Q4. Should ADMS information be reported for Animal Drug Availability Act (ADAA) feed use combinations of separately approved Type A medicated articles?
Q5. What types of establishments should be reported in ADMS?
Q6. Do contract testing laboratories need to be included in the ADMS book?
Q7. Does every supplier of an excipient, material, or component utilized in the manufacturing process need to be provided?
Q8. I manufacture a sterile drug substance or drug product. Do I need to include establishments with a Sterilizer Role?
Q9. Should I report Inactive establishments?
Q10. Should I report Withdrawn establishments?
Q11. When should I report establishments used solely for drug development?
Q12. What Supply Chain Entities are used in the ADMS system?
Q13. What Supply Chain Roles are used in the ADMS system?
Q14. What Supply Chain Qualifiers are used in the ADMS system?
Q15. What Operational Statuses are used in the ADMS system?
Q16. When should an approval submission be included in our ADMS information?
Q17. For the establishment contact information, should one list the US agent in the case of an establishment outside of the US or the contact at the actual site?
Q18. What data sources are available for establishment information?
Q21. What action should be taken when a submission acknowledgment contains the following message: “One or more ADMS establishment has invalid address.”
Q22. What action should be taken when a submission acknowledgment contains the following message: “Your submission has been rejected. The submission contains Master File Document ID(s) that do not match our records.”
Q23. When CVM returns ADMS information in an xml file, will that automatically update my ADMS Books?
Q24. How can an ADMS xml file sent from CVM be imported into an eSubmitter data book?
Submissions
Q1. What primary submission types may require ADMS Information?
| Submission Type | Doc. Type | Sub. Type | Class. Code |
|---|---|---|---|
| Major Technical Section Chemistry, Manufacturing and Controls | I/J | P | MC |
| Original (A)NADA | A/N | A | OT |
| Chemistry Annual Report1 | A/N | B | CA |
| Non-Fee Supplement2 | A/N | C | NF |
| Supplement3 | A/N | C | CI/CS/CP/AI/AS/AP |
| Supplement Containing Safety/Effectiveness | A/N | C | B1 |
| Quality Original, Total Update |
V | C | OT |
| Quality Annual Minor Changes and Stability |
V | C | AC |
| Quality Manufacturing/Chemistry Changes | V | C | MC |
1ADMS information is required in a Chemistry Annual Report submitted to an active drug product application. An application is considered active if the sponsor answers yes to any of the following questions listed in the eSubmitter template:
- Was the product manufactured during the reporting period?
- Was the product marketed during the reporting period?
- Does the application reference a Master File?
- Does the submission contain manufacturing changes?
- Does the submission contain updated stability data?
2ADMS information should be reported in an NF supplement if the sponsor answers no to the following question in the eSubmitter template and yes to any of the questions in footnote 3:
- Contain only changes to labeling language without supporting Chemistry, Manufacturing, and Controls (CMC) data?
3ADMS information should be reported in a supplement if the sponsor answers yes to any of the following questions in the eSubmitter template:
- Contain a new establishment for a drug substance, drug product, or novel or critical excipient?
- Add a new Role or Entity for an existing establishment?
- Contain a change to the manufacturing process at an approved facility?
- Contain Chemistry, Manufacturing, or Controls (CMC) data generated to support the change?
Q2. What types of submission reactivations may require ADMS information?
| Submission Type | Doc. Type | Sub. Type | Class. Code |
|---|---|---|---|
| Major Technical Section Chemistry, Manufacturing and Controls | I/J | P | MC |
| Original (A)NADA | A/N | E | OT |
| Chemistry Annual Report | A/N | F | CA |
| Non-Fee Supplement1 | A/N | R | NF |
| Supplement1 | A/N | R | CI/CS/CP/AI/AS/AP |
| Supplement Containing Safety/Effectiveness1 | A/N | R | B1 |
| Quality Manufacturing Response to Letter1 | V | C | MR |
1A reactivation of this submission should contain ADMS information if the primary submission contained ADMS information.
Q3. What primary submission types may not require ADMS Information?
| Submission Type | Doc. Type | Sub. Type | Class. Code |
|---|---|---|---|
| Chemistry Annual Report1 | A/N | B | CA |
| Supplement2 | A/N | C | NF |
| Supplement3 | A/N | C | CI/CS/CP/AI/AS/AP |
| Quality Manufacturing/Chemistry Changes | V | C | MC |
| Quality Manufacturing Response to Letter | V | C | MR |
1ADMS information is not required in a Chemistry Annual Report submitted to an inactive drug product application. An application is considered inactive if the sponsor answers no to all of the following questions listed in the eSubmitter template:
- Was the product manufactured during the reporting period?
- Was the product marketed during the reporting period?
- Does the application reference a Master File?
- Does the submission contain manufacturing changes?
- Does the submission contain updated stability data?
2ADMS information should not be reported in an NF supplement if the sponsor answers yes to the following question in the eSubmitter template:
- Contain only changes to labeling language without supporting Chemistry, Manufacturing, and Controls (CMC) data?
3ADMS information should not be reported in a supplement if the sponsor answers no to all of the following questions in the eSubmitter template:
- Contain a new establishment for a drug substance, drug product, or novel or critical excipient?
- Add a new Role or Entity for an existing establishment?
- Contain a change to the manufacturing process at an approved facility?
- Contain Chemistry, Manufacturing, or Controls (CMC) data generated to support the change?
Although the ADMS information is not always required for these submission types, the ADMS tab is still available. Minor changes or updates can be made to the ADMS information but CVM recommends that these types of changes be held until the next Chemistry Annual Report is submitted for that product. If the product is inactive, you may wait until the product is active again to submit updates to the ADMS information in the Chemistry Annual Report.
Q4. Should ADMS information be reported for Animal Drug Availability Act (ADAA) feed use combinations of separately approved Type A medicated articles?
CVM will collect ADMS information for each individually approved Type A medicated article application. CVM will not collect ADMS information for an ADAA combination application. An Chemistry Annual Report or Supplement submitted to an application for a Type A medicated article should not be linked to the referencing ADAA combinations.
Establishments
Q5. What types of establishments should be reported in ADMS?
For a submission to a drug product application or investigational file that should contain all establishments (e.g., a Major Technical Section Chemistry, Manufacturing and Controls [I/J-P-MC]):
- Report any establishment involved in the manufacture of the drug product.
- Example: manufacturer, packager, labeler, storer, sterilizer, and tester
- If a Type II master file (VMF or DMF) is referenced for drug substance manufacturing information:
- Report the drug substance manufacturer described in the master file.
- Do not report other supply chains described in the master file (e.g., drug substance packager or the master file holder’s drug substance tester).
- Report any establishment that performs confirmatory testing of the incoming drug substance for manufacturing the drug product.
- If a Type II master file (VMF or DMF) is not referenced for drug substance manufacturing (i.e., drug substance manufacturing information is reported in the drug product application or investigational file):
- Report any establishment involved in the manufacture of the drug substance.
- Example: manufacturer, packager, particle size reducer, and tester (release and/or stability)
- Report any establishment that performs confirmatory testing of the incoming drug substance for manufacturing the drug product.
- Do not report raw material suppliers or testers (solvents, reagents, and starting material)
- Report any establishment involved in the manufacture of the drug substance.
- Do not report establishments involved in the manufacture and/or testing of excipients unless the excipient is novel or critical. Changes to these categories of manufacturers or testers should still be described as part of Module 3 information.
- Do not report establishments involved in the manufacture and/or testing of packaging or components. Changes to these categories of manufacturers or testers should still be described as part of Module 3 information.
For a CMC supplement or NF supplement to an NADA or ANADA, the following questions are included in the eSubmitter template. If the sponsor answers yes to any of these questions, ADMS information should be provided. The types of establishments to report are listed below each question:
- Contain a new establishment for a drug substance, drug product, or novel or critical excipient?
- The currently approved establishment(s) and the proposed establishment should be reported.
Example #1: New drug product manufacturing establishment
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| Approved DP Manufacturer | Manufacturer/Drug Product | Approved Active1 |
| Proposed DP Manufacturer | Manufacturer/Drug Product | Pending |
| Proposed DP Manufacturer | Packager/Drug Product | Pending |
| Proposed DP Manufacturer | Labeler/Drug Product | Pending |
| Proposed DP Manufacturer | Tester/Drug Product/Chemical/Physical | Pending |
| Proposed DP Manufacturer | Tester/Drug Substance/Chemical/Physical | Pending |
1The operational status of the currently approved establishment should be updated appropriately. For example, if the currently approved establishment will not be manufacturing the drug product in the future, a status of Approved Inactive or Withdrawn would be appropriate.
- Add a new Role or Entity for an existing establishment?
- This establishment would be one previously approved for that application for a different Supply Chain Role/Entity. The existing establishment should be reported with the new Supply Chain Role/Entity information.
Example #2: Addition of a new Role to an existing establishment
In this example, the establishment was previously approved for chemical/physical testing of the drug substance but now is also proposed for microbiological testing of the drug product.
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| Approved DS Tester | Tester/ Drug Substance/ Chemical/Physical | Approved Active |
| Approved DS Tester | Tester/ Drug Product/ Microbiological | Pending |
- Contain a change to the manufacturing process at an approved facility?
- The approved establishment(s) where the change will be implemented should be reported.
Example #3: Change in proposed manufacturing process
In this example, the currently approved drug substance manufacturing facility is increasing the batch size.
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| Approved DS Manufacturer | Manufacturer/ Drug Substance/ Fermentation | Approved Active |
- Contain Chemistry, Manufacturing, or Controls (CMC) data generated to support the change?
- The establishment where the data was generated should be reported.
Example #4: CMC data generated to support a proposed change
In this example, the sponsor is proposing a change in the dissolution method that required the generation of new method validation data. This was performed at the contract test laboratory.
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| Approved Dissolution Tester | Tester/Drug Product/Chemical/Physical | Approved Active |
For an original or annual update submission to a veterinary master file, it should contain all establishments (e.g., a Quality, Original, Total Update [V-C-OT, V-C-AC]):
- Report any establishment involved in the manufacture of the drug substance.
- Example: manufacturer, packager, particle size reducer, and tester
- Report the establishment that manufactures the final intermediate as follows:
- Synthetic process: An intermediate manufacturer should be identified if different from the drug substance manufacturer.
- Semi-synthetic, fermentation, biotechnology products: An intermediate manufacturer may need to be identified if different from the drug substance manufacturer and depending on the process. You may contact CVM to discuss whether an intermediate manufacturer should be identified in the ADMS information.
- Do not report raw material suppliers or testers (solvents, reagents, and starting material).
For a Quality Manufacturing/Chemistry Changes submission to a veterinary master file [V-C-MC]:
- Report any establishment related to the change reported in the submission.
For a reactivation (e.g., Reactivation of Supplement) of a submission, follow the guideline above for the primary submission.
The examples below demonstrate the ADMS information that may be provided for a hypothetical drug product and drug substance.
Example #1: CMC technical section for a drug product that references a Type II master file for drug substance information
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| DP Manufacturer | Manufacturer/Drug Product | Pending |
| DP Manufacturer | Packager/Drug Product | Pending |
| DP Manufacturer | Labeler/Drug Product | Pending |
| DP Manufacturer | Storer/Drug Product | Pending |
| DP Manufacturer | Tester/Drug Product/Chemical/Physical | Pending |
| DP Manufacturer | Tester/Drug Product/Microbiological | Pending |
| DP Manufacturer | Tester/Drug Substance/Chemical/Physical | Pending |
| DS Manufacturer | Manufacturer/Drug Substance/Fermentation | Pending |
Example #2: VMF original submission for a micronized semi-synthetic drug substance
| Establishment | Role/Entity/Qualifier | Operational Status |
|---|---|---|
| DS Manufacturer | Manufacturer/Drug Substance/Semi-Synthetic | Active |
| DS Manufacturer | Packager/Drug Substance | Active |
| DS Manufacturer | Labeler/Drug Substance | Active |
| DS Manufacturer | Tester/Drug Substance/Chemical/Physical | Active |
| Intermediate Manufacturer | Manufacturer/Intermediate | Active |
| DP Micronizer | Particle Size Reducer/Drug Substance | Active |
Q6. Do contract testing laboratories need to be included in the ADMS book?
A contract testing laboratory should be reported to an application or investigational file if the laboratory performs testing of the drug product, incoming drug substance, or an excipient that is novel or critical. A contract testing laboratory should be reported to a master file if the laboratory performs testing of the finished drug substance.
Q7. Does every supplier of an excipient, material, or component utilized in the manufacturing process need to be provided?
There are certain categories of establishments that CVM has determined will not be collected at this time.
In most cases, ADMS information should not be reported for the manufacture and/or testing of excipients in a drug product application. If the excipient is novel or critical, ADMS information should be reported for any suppliers.
Establishment information for raw material suppliers or testers (solvents, reagents, and starting material) is also not included for drug substance manufacturing supply chain information.
ADMS information should not be reported for packaging or component suppliers.
Note that changes to these categories of manufacturers or testers should still be described as part of Module 3 information.
Q8. I manufacture a sterile drug substance or drug product. Do I need to include establishments with a Sterilizer Role?
Yes. Any drug product or drug substance that is labeled as sterile should include at least one establishment identified as performing the Sterilizer function, regardless of the sterilization method (e.g., aseptic or terminal).
Q9. Should I report Inactive establishments?
If an establishment is reported to a drug product application with the “Approved Active” or to a master file with the “Active” operational status, the application should be actively maintained. For active establishments, the sponsor is expected to assess changes at the establishment, communicate with suppliers, assess changes in any referenced master files, etc.
Report all other approved establishments that are not currently in use but may be used in the future. “Approved Inactive” or “Inactive” may be an appropriate operational status in this case. “Approved Inactive” (applications) or “Inactive” (master files) is appropriate for establishments that are approved for use for that role, is not used to manufacture/market product, and the application/file may not be actively maintained, including any referenced master files.
If an establishment was previously approved, is not currently in use but still has ongoing studies (e.g., stability lots), either “Approved Active” or “Approved Inactive” could be appropriate. The Establishment Activities Performed field should be updated to reflect the current situation to provide context to the reviewer.
Q10. Should I report Withdrawn establishments?
The Withdrawn operational status should be applied to an establishment that you no longer wish to maintain in the application or file including establishments that are no longer registered with FDA, no longer capable of performing its approved role, or you have no intention of using this facility in the future.
If you have not provided previously reported ADMS information for a withdrawn establishment, it does not need to be reported as “Withdrawn”.
To withdraw an establishment that was previously reported as “Approved Active,” “Approved Inactive,” “Active,” or “Inactive”, report the establishment with the “Withdrawn” operational status. After an established is reported as “Withdrawn,” it does not need to be reported again.
Q11. When should I report establishments used solely for drug development?
Establishments used solely for drug development should be reported for investigational (J)INAD files if data were generated at that establishment to support approval of the product.
Generally, establishments used solely for drug development should only be reported for supplemental changes (CMC or NF supplements) if data to support the supplement were generated at an establishment that is not intended to be used for commercial operations.
Establishments used solely for drug development should be reported for VMF original (C-OT) submissions for any establishments that were used to generate laboratory or pilot scale batches used to support studies.
Establishments used solely for drug development should not be reported to Chemistry Annual Report, administrative (A)NADAs, or B1 supplements.
ADMS data elements
Q12. What Supply Chain Entities are used in the ADMS system?
| Entity | Description |
|---|---|
| Drug Product | A finished dosage form, for example, tablet, capsule, or solution, etc., or Type A medicated article that contains one or more active ingredients (or drug substance) generally, but not necessarily, in association with inactive ingredients (21 CFR 210.3(b)(4)). |
| Drug Substance | An active ingredient that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the human body, does not include intermediates used in the synthesis of such ingredient (21 CFR 314.3(b)). The term drug substance can also be used to refer to a physical mixture of two or more drug substances used to produce a fixed-combination drug product. |
| Excipient | Any intended component of the drug other than drug substance. |
| Intermediate | A material produced after the starting material but prior to the synthesis of the drug substance. |
| Other | Any other Entity not described elsewhere, for example, a proprietary Type B medicated feed or proprietary Type C medicated feed (including Type C free-choice medicated feed) approved under its own application or approved under a Type A medicated article application. |
Q13. What Supply Chain Roles are used in the ADMS system?
| Role | Description |
|---|---|
| Labeler | A site that affixes an identifier to a substance or the container for the substance. |
| Manufacturer | A site that produces a substance or component. Note that a manufacturer commonly performs multiple roles such as labeler, packager, etc. Each of these additional roles, if performed by the same establishment for this file or application, should be provided as a separate Supply Chain item. |
| Other | Any site that performs an activity not identified by one of the other Roles. |
| Packager | A site that places a substance into a primary container (e.g., has direct contact with the substance). |
| Particle size reducer | A site that breaks particles of a substance into smaller pieces using a mechanical process (e.g., milling). |
| Relabeler | A site that adds or replaces the original identifier on a substance or the container for the substance. |
| Repackager | A site that removes a substance from the primary container and places it into a new container. |
| Secondary packager | A site that performs activities related to placing the primary container into additional packaging. |
| Sterilizer1 | A site that uses a validated process to render a substance or component free from viable microorganisms. |
| Storer | A site that warehouses samples of substances for stability analysis. |
| Tester | A site that analyzes one or more characteristics and/or attributes of a substance or component. |
1This supply chain role may be appropriate for aseptic processing and terminal sterilization.
Q14. What Supply Chain Qualifiers are used in the ADMS system?
For supply chains that include the Tester role, one of the following qualifiers must be selected:
| Qualifier | Description |
|---|---|
| In-process | Sites that perform testing of the characteristics and attributes of the drug substance or drug product during the manufacturing process. |
| Chemical/Physical | Sites that perform chemical or physical testing of the characteristics and attributes of drug substances or drug products such as assay, dissolution, etc. |
| Microbiological | Sites that perform microbiological testing of the characteristics and attributes of drug substances or drug products such as sterility, endotoxin, etc. |
| Biological | Sites that perform biological testing of the characteristics and attributes of drug substances or drug products such as rat bioassay. |
For supply chains that include the Drug Substance entity and Manufacturer role, one of the following qualifiers must be selected:
| Qualifier | Description |
|---|---|
| Synthetic | The production of drug substances from chemical reactions. |
| Fermentation | The production of drug substances derived from the culture of microorganisms. |
| Semi-synthetic | The production of drug substances from a combination of chemical reactions and the culture of microorganisms. |
| Biotechnology | The production of drug substances from raw materials using cell culture, peptide synthesis, or other biotechnology-related processes. |
| Other | The production of drug substances from raw materials using processes not otherwise defined. This may include processes such as biological extractions. |
Q15. What Operational Statuses are used in the ADMS system?
For drug production applications and investigational files, the following operational statuses are used in the ADMS system:
| Status | Description |
|---|---|
| Pending | This status should be applied to a new establishment added to the application or file or a new supply chain role for an approved establishment. |
| Approved Active |
This status should be applied to an establishment that meets each of the following criteria:
|
| Approved Inactive |
This status should be applied to an establishment that you wish to maintain in the application or file that meets each of the following criteria:
|
| Withdrawn | This status should be applied to an establishment that you no longer wish to maintain in the application or file including establishments that are no longer registered with FDA, no longer capable of performing its approved role, or you have no intention of using this facility in the future. |
| CBE EST IMPL | An establishment that may be used at risk in the manufacture of a drug substance or drug product but is pending approval. This status cannot be selected by a firm in eSubmitter unless CVM has previously returned this status after a CBE-0 or CBE-30 submission was determined to be incomplete. |
For veterinary master files, the following operational statuses are used in the ADMS system:
| Status | Description |
|---|---|
| Active | This status should be applied to an establishment that is or could be used to manufacture/market the drug substance. |
| Inactive | This status should be applied to an establishment that is not used to manufacture/market the drug substance. |
| Withdrawn | This status should be applied to an establishment that you no longer wish to maintain in the application or file including establishments that are no longer registered with FDA, no longer capable of performing its approved role, or you have no intention of using this facility in the future. |
Q16. When should an approval submission be included in our ADMS information?
If the establishment was previously approved, an approval submission ID should be provided for each supply chain associated with that establishment. CVM recognizes that this information may be challenging to locate; therefore, a value is not required to be able to submit ADMS information to CVM. CVM requests that you make every effort to determine the approval submission ID for each establishment associated with the application. If the approval submission ID cannot be located, any information you may have such as the date of approval or a date range during which approval may have been received will aid CVM in identifying the submission where approval was granted.
Approval submissions IDs are not required for ADMS information submitted to master files because master file submissions are not approved.
Q17. For the establishment contact information, should one list the US agent in the case of an establishment outside of the US or the contact at the actual site?
An onsite contact at the facility is recommended. US Agents are required for sponsors of applications or master files outside the US. If an onsite contact is not available for a facility located outside the US, a US Agent may be used.
Q18. What data sources are available for establishment information?
The FDA Inspections Data Dashboard (https://datadashboard.fda.gov/ora/cd/inspections.htm) is available to the public and contains firm name, address, FEI number, and inspection information.
The Drug Establishments Current Registration Site (https://www.accessdata.fda.gov/scripts/cder/drls/default.cfm) is available to the public and contains firm name, address, FEI number, DUNS number, and Business Operations information.
The Dun & Bradstreet D-U-N-S® Number Lookup (https://www.dnb.com/duns-number/lookup.html) is available to the public and contains firm name, address, and DUNS number information for US companies.
The Dun & Bradstreet Import Safety Lookup Portal (https://importregistration.dnb.com/Login.aspx) is available to the public (registration required) and contains firm name, address, and DUNS number information for US and non-US companies.
The FEI Search Portal (https://www.accessdata.fda.gov/scripts/feiportal/index.cfm) is available to the public (registration required) and contains firm name, address, and FEI number information.
Entering ADMS data
Q19. CVM has asked for additional supply chains to be added to an existing establishment. How can another supply chain be added to an establishment?
- Open the eSubmitter ADMS Supply Chains book
- Open an existing supply chain.
- Click Add Another Role…
- Select the New Role and Qualifier.
- Click OK.
- Select the Operational Status.
- Add the Approved Submission ID, if appropriate.
- Verify the information.
- Click OK to save.
Q20. How can data from an ADMS excel file be imported into an eSubmitter data book?
ADMS establishment and supply chain information can be stored in an Excel spreadsheet and imported into the ADMS books in eSubmitter. A sample Excel spreadsheet is available in the eSubmitter application import folder. Additional information on importing ADMS data from an Excel spreadsheet is provided in the CVM eSubmitter Animal Drug and Manufacturing (ADMS) Book Quick Guide.
CVM Communications
Q21. What action should be taken when a submission acknowledgment contains the following message: “One or more ADMS establishment has invalid address.”
A submission acknowledgment may contain the following message: “One or more ADMS establishment has invalid address.” No immediate action by the firm is required in response to this message. If additional ADMS information is required, the reviewer will contact the responsible official.
Q22. What action should be taken when a submission acknowledgment contains the following message: “Your submission has been rejected. The submission contains Master File Document ID(s) that do not match our records.”
A submission acknowledgment may contain the following message: “Your submission has been rejected. The submission contains Master File Document ID(s) that do not match our records.” No immediate action by the firm is required in response to this message. The submission will not be rejected for having a Master File Document ID(s) that does not match our records. This is typically caused by identifying a VMF as a DMF or vice versa. If additional ADMS information is required, the reviewer will contact the responsible official.
Q23. When CVM returns ADMS information in an xml file, will that automatically update my ADMS Books?
CVM will return ADMS information in an xml file, but the eSubmitter books will not be updated automatically. The xml file should be imported into eSubmitter using the instructions provided in Question 24.
Q24. How can an ADMS xml file sent from CVM be imported into an eSubmitter data book?
Download the zip file containing the CVM response received through the ESG when the review was completed.
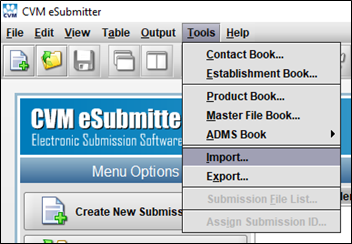
- In eSubmitter, click “Import” in the “Tools” menu.
- Select the Import Type “CVM Response Acknowledgement (zip file) into ADMS Book”.
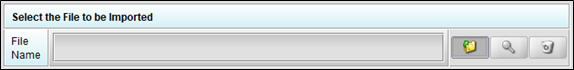
- Click the “Select File” button.
-
Navigate to the folder containing the zip file, select the file, and click the “Select” button.
-
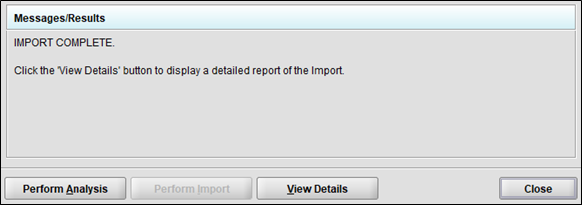
Click the “Perform Analysis” button to see a summary of the changes that will be made to the ADMS books.
-
Click the “Perform Import” button to make the changes to the ADMS books.