FDA Regulated Meats and Meat Products for Human Consumption
Meat and meat products are regulated by both the U.S. Food and Drug administration (FDA) and the United States Department of Agriculture (USDA). The USDA Food Safety and Inspection Service (FSIS) has primary responsibility for regulating meat from the species of animals listed in the Federal Meat Inspection Act and the Poultry Products Inspection Act. These animals are considered “amenable species” and include cattle, sheep, swine, goats, domestic poultry (chickens, turkeys, ducks, geese, and guinea), ratites, and squab.
The FDA regulates game meats and game meat products, referred to as “non-amenable” meats. These animals include antelope, bison, deer, elk, reindeer, muskrat, non-aquatic reptiles, opossum, rabbit, raccoon, squirrel, water buffalo, grouse, pheasant, quail, wild turkey, wild geese, and wild ducks.
Information for Industry
Non-amenable meats and meat products are from animals and birds that are reared, slaughtered, and commercially sold for food. The 2022 Food Code (3-201.17) generally provides for food establishments to use game meat that is processed under a voluntary inspection program or through a regular inspection program, as allowed by law. All non-amenable meat and meat products must meet the FDA’s requirements, including the FDA’s labeling requirements for packaged foods.
The USDA Food Safety Inspection Service can perform voluntary inspections (fee for service) for certain non-amenable species, under the Agricultural Marketing Act of 1946. More information can be found at Voluntary and Other Reimbursable Inspection Services. FDA-regulated, or non-amenable, meats that are slaughtered under voluntary inspection of USDA FSIS may receive a USDA FSIS voluntary mark of inspection.
Meat and meat products from animals diagnosed with a disease may be considered adulterated under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and, if so, may not be sold in interstate commerce.
Import Requirements
FDA-regulated, or non-amenable, meats and meat products imported to the US from other countries must meet the same FDA safety standards applied to all foods domestically produced and offered for entry into U.S. interstate commerce. Companies exporting FDA-regulated meat to the U.S. must comply with all applicable FDA regulations, including registration and prior notice requirements. Any imported food under FDA jurisdiction must be safe, wholesome, properly labelled and fully compliant with all applicable FDA requirements. Domestic and international food shipments found not to comply with the provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act) must be brought into compliance, destroyed, or if from other countries, may be re-exported.
The FDA does not require a license to import food. However, as mentioned above, the FDA requires the registration of food facilities that export foods to the U.S., and prior notification of food (including animal feed) that is imported or offered for import into the United States. The FDA may inspect foreign facilities, and the FD&C Act provides that the FDA shall refuse admission of a food from a foreign establishment of which the owner, operator, or agent in charge, or the government of the foreign country, refuses to permit entry of FDA investigators. In addition, shipments are evaluated at entry by U.S. Customs and Border Protection on behalf of the FDA and are subject to examination at entry by the FDA.
The United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) may have additional requirements for meat offered for import into the United States. APHIS monitors the importation into the U.S. of meat and poultry to prevent infectious disease spread by animals. Additionally, some U.S. states may have specific requirements for importation into their jurisdiction. The importer or U.S. agent for the firm may be able to provide additional information regarding specific U.S. state requirements and/or APHIS requirements.
Relevant Regulations and References
- Federal Food, Drug, and Cosmetic Act
- Food Industry
- FDA Food Code 2022
- Registration of Food Facilities and Other Submissions
- CPG Sec 565.100 FDA Jurisdiction Over Meat and Poultry Products
- USDA Food Safety and Inspection Service
- Federal Meat Inspection Act
- Poultry Products Inspection Act
- Voluntary and Other Reimbursable Inspection Services
- USDA Food Safety and Inspection Service - Food Standards and Labeling Policy Book
- USDA APHIS
- Human Food Made with Cultured Animal Cells
- Outbreak Investigation of E. coli: Ground Bison (July 2019)
Section 1 - Meats
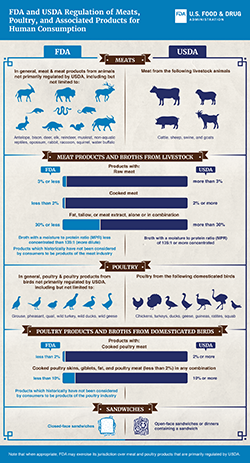
FDA, in general, regulates meat and meat products from animals not primarily regulated by USDA, including but not limited to antelope, bison, deer, elk, reindeer, muskrat, non-aquatic reptiles, opossum, rabbit, squirrel, and water buffalo.
USDA regulates meat from livestock, which includes cattle, sheep, swine, and goats.
Section 2 – Meat Products
FDA and USDA divide primary regulation of meat products and broths made from livestock (cattle, sheep, swine, and goats) based on the amount of meat or other animal tissue in the product. FDA regulates meat products that contain 3% or less raw meat, less than 2% cooked meat, or 30% or less fat, tallow, or meat extract, alone or in combination. USDA regulates meat products that contain more than 3% raw meat, 2% or more cooked meat, or more than 30% fat, tallow, or meat extract, alone or in combination. FDA regulates meat-based broths with a moisture to protein ratio (MPR) of less than 135:1 (i.e. more dilute). USDA regulates meat-based broths with an MPR of 135:1 or greater (i.e. more concentrated). FDA regulates products which historically have not been considered by consumers to be products of the meat industry.
Section 3 – Poultry and Poultry Products
FDA, in general, regulates poultry and poultry products from birds not primarily regulated by USDA, including but not limited to grouse, pheasant, quail, wild turkey, wild ducks, and wild geese.
USDA regulates poultry from domesticated birds, which include chickens, turkeys, ducks, geese, guineas, ratites, and squab.
FDA and USDA divide primary regulation of poultry products and broths made from domesticated birds based on the amount of poultry meat or other poultry tissue in the product. FDA regulates poultry products that contain less than 2% cooked poultry meat or less than 10% cooked poultry skins, giblets, fat, and poultry meat (less than 2%) in any combination. USDA regulates poultry products that contain 2% or more cooked poultry meat or 10% or more cooked poultry skins, giblets, fat, and poultry meat (less than 2%) in any combination. FDA regulates products which historically have not been considered by consumers to be products of the poultry industry.
Section 4 – Sandwiches
FDA and USDA divide primary regulation of sandwiches containing meat or poultry. FDA regulates closed-face sandwiches, unless it is a part of a dinner. USDA regulates open-face sandwiches or dinners containing a sandwich.
Section 5 – Note at Bottom
Note that when appropriate, FDA may exercise its jurisdiction over meat and poultry products that are primarily regulated by USDA.