What We Do
Here in the FDA Innovation Lab, we use a variety of Innovative Specialists and Tools. Innovation Specialists work on ideas from within the Innovation Lab and the FDA Community to perform technological surveillance and generate prototypes, pilots, proof of concept and whitepapers on topics ranging from Machine Learning, Deep Learning, Cloud Computing, High Performance Computing, to Data Masking, Mobile applications and many more. There is always something fun and exciting happening in the lab, whether it’s creating Dashboards using the latest Data Analytic tools, testing out the VGO Robot, building something on the 3D printer, or hosting a Workshop regarding a popular technology.
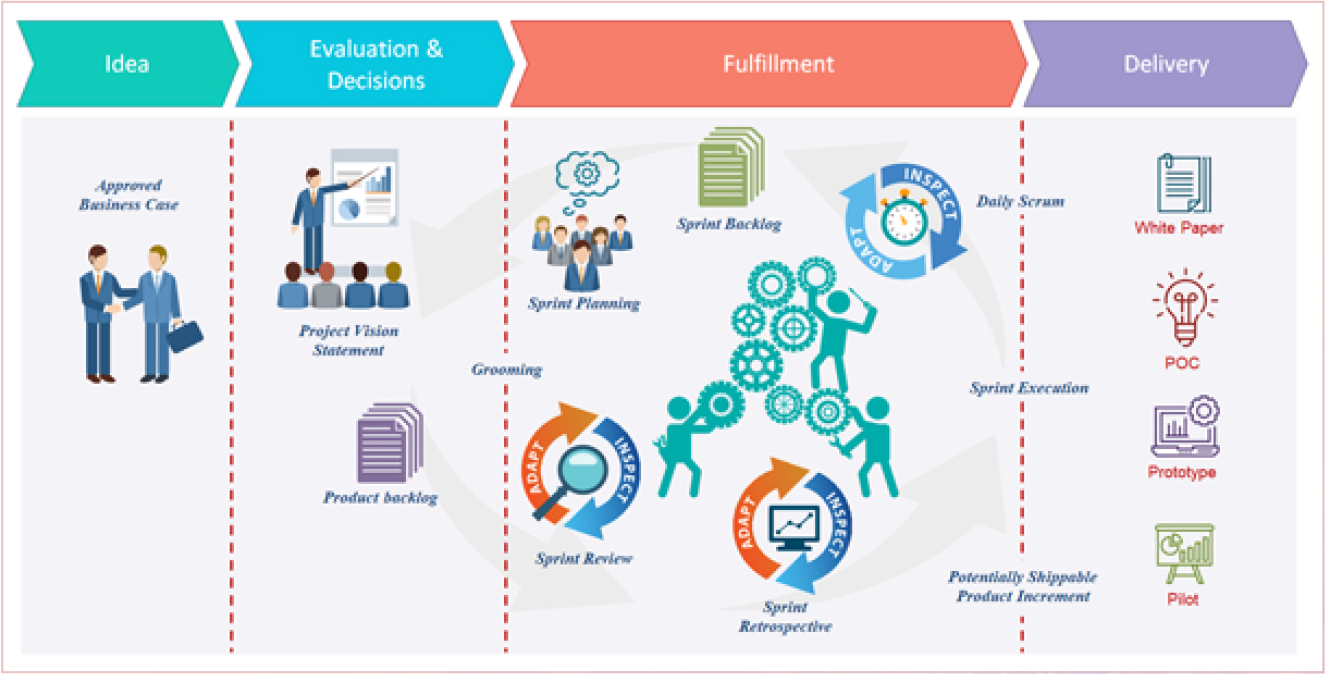
Whitepaper: A White Paper is an informative write up about a technology or philosophy. It informs concisely about a complex issue, advancing understanding for better informed decisions.
Proof of Concept: A proof of concept (POC) is a demonstration, the purpose of which is to verify that certain concepts or theories have the potential for real-world application. POC is therefore a prototype that is designed to determine feasibility, but does not represent deliverables.
Prototype: A prototype is an original model, form or an instance that serves as a basis for other processes. A prototype helps support a proof of concept in a tangible way. In software technology, the term prototype is a working example through which a new model or a new version of an existing product can be derived.
Pilot: A Pilot is used for an isolated roll out of software in real world conditions, but to a smaller set of users. Its purpose is testing software, as it will function in production, before being released to an entire organization, thus minimizing risk. It can also be used to provide initial exposure to users for educational purposes before full online implementation.
We welcome Innovation at the FDA through various channels like Tech Talkx, Workshops, Ask the Expert and by encouraging FDA resources to use the Idea Exchange to express innovative ideas.
Ask the Expert: The Ask the Expert event series allows the FDA or Innovation Industry expert an hour of time to share their vision with FDA employees and contractors. It also allows for the attendees to ask questions of these executive-level individuals.
TechTalkx: TechTalkx are technical discussions that are specific to a particular technology or new innovation. The audience for these one hour discussions tends to be more technical and hands on with the solutions.
Workshops: Workshops which are hands-on demonstrations of new innovative technologies that may be considerations for use within the FDA.
Idea Exchange: Ideas can be submitted by anyone within the FDA community; these are reviewed by the Innovation Lab experts and selected based on defined acceptance criteria to be considered for further analysis.
HOW WE DO IT
FDA’s capacity for innovation commences with a well thought out innovation approach. A coherent set of interdependent steps that governs how the Office of Innovation searches for novel problems and solutions forms the Innovation Approach. FDA Innovation Lab conducts technology surveillances and synthesizes ideas into a business concept or product design (intake), selects which ideas are to be implemented (assessment, evaluation and decision) and implements the selected ideas using the appropriate technology (fulfilment). The outcome of the fulfilment phase is a prototype, a proof of concept, a white paper or a pilot.