Closer to Zero: Action Plan for Baby Foods
About | Approach | Action Items & Proposed Timeline | On-Going Work | Announcements
Featured
November 18, from 10:00 a.m. to 4:00 p.m. (EST)
About the Action Plan
The U.S. Food and Drug Administration’s (FDA) plan, Closer to Zero, identifies actions the agency will take to reduce exposure to toxic elements from foods eaten by babies and young children—to as low as possible. We have prioritized babies and young children because their smaller body sizes and metabolism make them more vulnerable to the harmful effects of these contaminants.
Exposure to toxic elements, including arsenic, lead, cadmium, and mercury, from foods depends on the levels of the elements in the food and the amount consumed. The levels of toxic elements in foods depend on many factors, including:
- the levels of these elements in the air, water, and soil used to grow the crops, which vary depending on factors such as natural geographical differences and past or current contamination,
- the type of food crop and how much “uptake” there is of specific elements from the environment, and
- industrial, manufacturing, and agricultural processes.
Our work, combined with that of our stakeholders, has led to meaningful reductions in exposure to toxic elements. The FDA’s action plan builds on this progress and outlines a science-based, iterative approach for achieving continual improvements over time. Further reductions in the levels of toxic elements in foods will be made by:
- advancing the FDA’s research on and evaluating changes in dietary exposures to toxic elements,
- setting action levels, with input from stakeholders,
- encouraging adoption of best practices by industry to lower levels of toxic elements in agricultural commodities and products,
- increasing targeted compliance and enforcement activities, and
- monitoring progress of levels over time.
Action levels are a level of contamination at which a food may be regarded as adulterated within the meaning of section 402(a)(1) of the Federal Food, Drug, and Cosmetic Act. The FDA considers action levels, in addition to other factors and scientific evidence, when considering whether to bring enforcement action in a particular case.
Reducing levels of toxic elements in foods is complicated and multifaceted. It is crucial to ensure that measures taken to limit toxic elements in foods do not have unintended consequences—like eliminating from the marketplace foods that have significant nutritional benefits or reducing the presence of one toxic element while increasing another.
The FDA is committed to a science-driven, transparent, and inclusive process that will include active stakeholder engagement and public sharing of data and information. This iterative plan will be updated as new data, information, and resources become available.
Understanding the FDA’s Approach
To make continual improvements, the FDA’s action plan follows a four-stage iterative approach that includes research, regulatory, and outreach efforts.
The Four Stages of the FDA’s Approach:
Evaluate the scientific basis for action levels. The cycle of continual improvement starts with the FDA evaluating existing data from routine testing of the food supply, research and data on chemical analytical methods, toxicological assays, exposure and risk assessments, and other relevant scientific information. Through a process that may include engagement with stakeholders, advisory committees, public workshops, and consultation with scientific experts, federal agency partners and other stakeholders, the agency will establish interim reference levels (IRLs) for certain toxic elements as appropriate. An IRL is a measure of exposure from food that the FDA may use to determine if the amount of exposure to an individual element across foods could result in a specific health impact.
Propose action levels. The IRLs may be among the key factors that inform the development of the FDA’s proposed action levels for certain toxic elements in categories of baby foods (e.g., cereals, infant formula, pureed fruits and vegetables) and other foods commonly eaten by babies and young children.
Consult with stakeholders on proposed action levels, including the achievability and feasibility of action levels. For each toxic element in every identified category of food, the FDA will gather data and other information through a process of consultation which could include workshops, scientific meetings, and collaboration with federal partners to assess, among other things, the achievability and feasibility of the proposed action levels and the timeframes for reaching them.
Finalize action levels. The FDA will use the information gathered from stakeholders, updated scientific research, and routine monitoring of data to make any needed adjustments and finalize action levels.
The FDA's Approach in Action
Once the FDA has published final action levels, the agency will establish a timeframe for assessing industry’s progress toward meeting the action levels and recommence the cycle to determine if the scientific data support efforts to further adjust the action levels.
The availability of data and additional research needs for arsenic, lead, cadmium, and mercury are different. Therefore, because of currently available data, we will start with proposing action levels for lead while we evaluate data for the other toxic elements. We will then progress through the cycle for each element as we gather more data and information across various categories of food consumed by babies and young children.
The FDA will support the cycle of continual improvement with ongoing activities related to monitoring levels in foods; research on chemical methods, toxicological impacts, exposures, and risk communication; and routine compliance and enforcement efforts. The FDA will evaluate information as it becomes available to determine whether any foods are adulterated or otherwise violative and take action as appropriate. The FDA intends to make information about our monitoring, research, and enforcement actions publicly available. The timeline and deliverables included in the plan will be updated periodically as new data, information, and resources become available.
The FDA’s Approach for Setting Action Levels for Lead
The Centers for Disease Control and Prevention (CDC) has identified a blood reference level of 5 micrograms of lead per deciliter of whole blood (ug/dL) as the level at which they recommend clinical monitoring of lead exposure in children. Using the CDC’s level as a biomarker, in 2018 the FDA calculated a maximum daily intake for lead from food, termed the interim reference level (IRL). The IRL is the calculated amount of dietary lead intake that would be required to reach the CDC’s blood reference level, including a 10x safety factor. The calculated IRLs are 3 micrograms (µg) per day for children and 12.5 µg per day for adults.
The adult level is particularly important for women of childbearing age, given concerns about possible fetal exposure in women who are unaware that they are pregnant and to protect against infant exposure during nursing.
In Phase 1 of the FDA’s Action Plan, we will propose a set of action levels for lead in certain foods eaten by babies and young children, considering the IRLs for dietary lead we have published and other data. We will seek data and other information from stakeholders on issues related to the feasibility of achieving those levels, among other things. In Phase 2, we will adjust as appropriate and finalize the proposed action levels. After a period of monitoring that may also include enforcement, we will reassess as part of Phase 3 whether those levels should be adjusted downward.
Action Items and Proposed Timeline
Phase 1: April 2021 – April 2022
|
Stage in the Cycle of Improvement |
Toxic Element |
Action |
|---|---|---|
|
|
Arsenic | Gather data through a process of consultation, which could include workshops, scientific meetings, and collaboration with federal partners to work toward establishing an interim reference level |
|
|
Lead | Draft action levels for categories of foods consumed by babies and young children |
|
|
Lead | Engage with stakeholders to assess, among other things, feasibility and best practices |
|
|
All |
Provide resources to industry on best practices for reducing or preventing lead contamination Complete sampling assignment for baby foods |
Phase 2: April 2022 – April 2024
|
Stage in the Cycle of Improvement |
Toxic Element |
Action |
|---|---|---|
|
|
Cadmium Mercury |
Gather data through a process of consultation which could include workshops, scientific meetings, and collaboration with federal partners to work toward establishing an interim reference level |
|
|
Arsenic | Draft action levels for categories of foods consumed by babies and young children |
|
|
Arsenic | Engage with stakeholders to assess, among other things, feasibility and best practices |
|
|
Lead | Finalize action levels for lead in categories of foods consumed by babies and young children |
|
|
All |
Issue guidance chapter on chemical hazards in the Draft Guidance for Industry on Hazard Analysis and Risk-Based Preventive Controls for Human Food Publish results from sampling assignment for baby foods Publish Total Diet Study results (to occur biennially) Publish exposure assessments for children to toxic elements (to occur biennially) |
Phase 3: April 2024 – beyond
|
Stage in the Cycle of Improvement |
Toxic Element |
Action |
|---|---|---|
|
|
Lead | Review new scientific data, assess progress on reducing lead in foods consumed by babies and young children and feasibility of attaining even lower levels |
|
|
Cadmium Mercury |
Draft action levels for categories of foods consumed by babies and young children |
|
|
Cadmium Mercury |
Engage with stakeholders to assess, among other things, feasibility and best practices |
|
|
Arsenic | Finalize action levels for arsenic in categories of foods consumed by babies and young children |
|
|
All |
Complete additional baby food sampling assignments Publish results from sampling assignment for baby foods Publish Total Diet Study results (to occur biennially) Publish exposure assessments for children to toxic elements (to occur biennially) |
As action levels are finalized, we will continue the cycle of continual improvement, addressing each toxic element in its turn, to evaluate whether downward adjustments of interim reference levels should be made; proposing new action levels, as appropriate; consulting with stakeholders on feasibility, achievability, and other issues; and adjusting (as needed) and finalizing action levels.
Ongoing Work on Toxic Elements
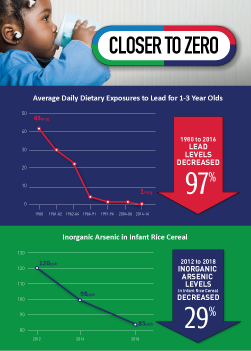
The FDA’s testing of toxic elements in foods has shown that over time there have been significant reductions in the levels of toxic elements in foods.
The action plan will help further advance our work in this area. In addition, we will continue our research and collaborations that are ongoing and our work to set action levels in juices, including:
- Finalizing action levels for arsenic in apple juice and issuing draft action levels for lead in other juices.
- Continuing development and validation of analytical methods.
- Continuing toxicological research on impacts of toxic elements on development in children.
- Developing new dose-response models to consider the probability of other adverse health effects in different sub-populations, including infants and young children.
- Collaborating with the U.S. Department of Agriculture on research on agronomic techniques that may mitigate uptake of toxic elements in agricultural commodities.
- Collaborating with the National Institutes of Health and the Centers for Disease Control and Prevention to better understand impacts of toxic elements on development and the role of nutrition for mitigating those impacts.
- Evaluating potential impact of new technologies, interventions, or mitigation controls to reduce exposure and resulting risk to consumer health.
- Reevaluating risk assessments based on declining levels of toxic elements in foods.
Announcements
FDA Announces First Closer to Zero Action Plan Public Meeting (October 8, 2021)
FDA Statement: FDA Releases Action Plan for Reducing Exposure to Toxic Elements from Foods for Babies, Young Children (April 8, 2021)
FDA Shares Action Plan for Reducing Exposure to Toxic Elements from Foods for Babies and Young Children (April 8, 2021)
FDA Statement: FDA Announces New Actions Aimed at Further Reducing Toxic Elements in Food for Babies, Young Children (March 5, 2021)
FDA Response to Questions About Levels of Toxic Elements in Baby Food, Following Congressional Report (February 16, 2021)