FY2016 Regulatory Science Report: Long-Acting Injectable Formulations
This section contains only new information from FY2016. For background scientific information and outcomes from previous years on this research topic, please refer to the FY15 Regulatory Science Research Report on Long-Acting Injectable Formulations.
Introduction
Long-acting injectable (LAI) formulations include biodegradable injectable microspheres and in-situ gelling implants. Compendial in vitro release methods for these complex formulations are not well developed, and demonstration of BE for these products can be challenging. Therefore, having a better understanding of the key factors which impact in vitro drug release kinetics and in vitro in vivo correlations (IVIVCs) may aid in the development of future recommendations including in vitro approaches.
Research
Currently, there are 7 ongoing research projects in the area of long-acting injectable formulations including 5 research grants and two BAA contracts. The focus of these projects are:1) to explore biorelevant IVIVCs for biodegradable injectable poly lactide-co-glycolide (PLGA) microspheres (two research grants); 2) to investigate dissolution methods for PLGA microsphere and implant drug products (two grants); 3) to obtain a better understanding of the impact of properties of PLGA polymers on product performance (one BAA contract), 4) to develop modeling tools to facilitate development of generic LAI formulation development as well as bioequivalence guidances for LAI formulations (one BAA contract); and 5) to investigate potential peptide PLGA interactions during product manufacturing and use (one research grant).
The most significant findings to date are:
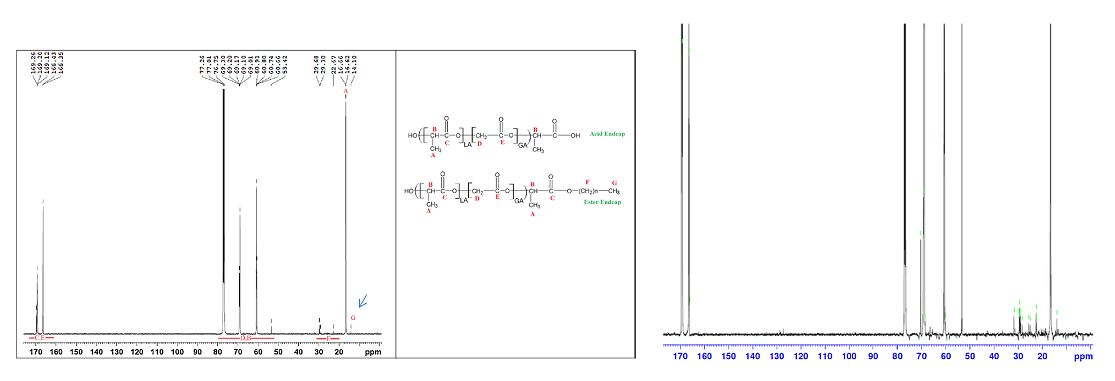
1) An analytical protocol describes methods for characterizing key physicochemical properties of poly(lactide-co-glycolide) polymers in commercial LAI formulations has been developed and published. Treslstar® and Risperdal® Consta® are used as model products and Figure 1 shows the 13C NMR spectra of PLGA used in these two products indicating both PLGAs have the ester end cap.
Figure 1 Left: 13C NMR sepctra of purified PLGA from Treslstar® and resultant peak assignments. Right: 13C NMR sepctra of purified PLGA from Risperdal® Consta® (Reference 1).
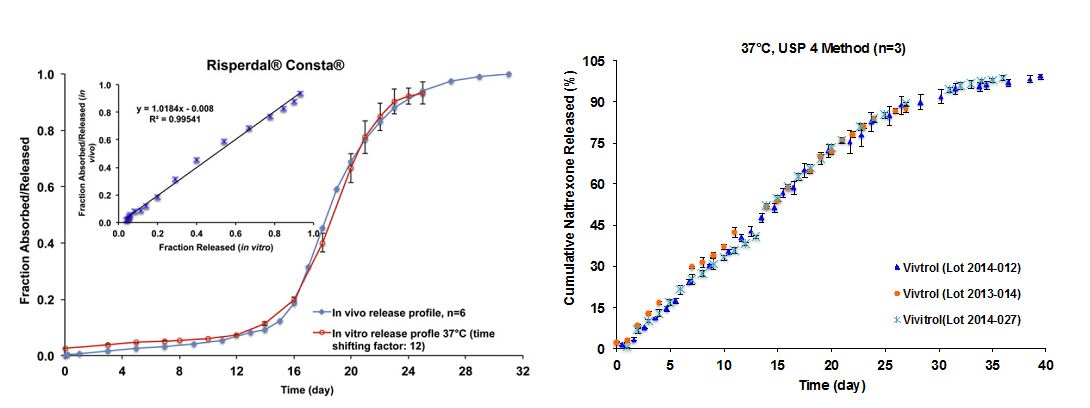
2) Level A type of IVIVCs have been established for risperidone microspheres and naltrexone microspheres using a rabbit model (Figure 2). All in house developed test products are similar in composition to the approved formulations. Analysis of critical physicochemical properties showed that risperidone microspheres that are similarly composed are very sensitive to manufacturing differences. An in vitro release test using USP apparatus 4 demonstrated excellent discriminatory ability and may also have the potential to predict the in vivo performance of these microspheres.
Figure 2 Left (Reference 2): In vivo absortpion/release and in vitro release (time shifting factor: 12) profiles in 10 mM PBS (pH 7.4) at 37ºC of Risperdal® Consta®. Inserted figure shows linear correlation between fractions released in vitro (37 °C) and fraction absorbed/released in vivo. Right (courtesy of Dr. Diane Burgess): In vitro release profiles of three batches of Vivitrol® obtained using USP apparatus 4 in 10 mM PBS (pH 7.4) with 0.02% (v/v) Tween 20 and 0.02% (w/v) sodium azide at 37°C (n=3).
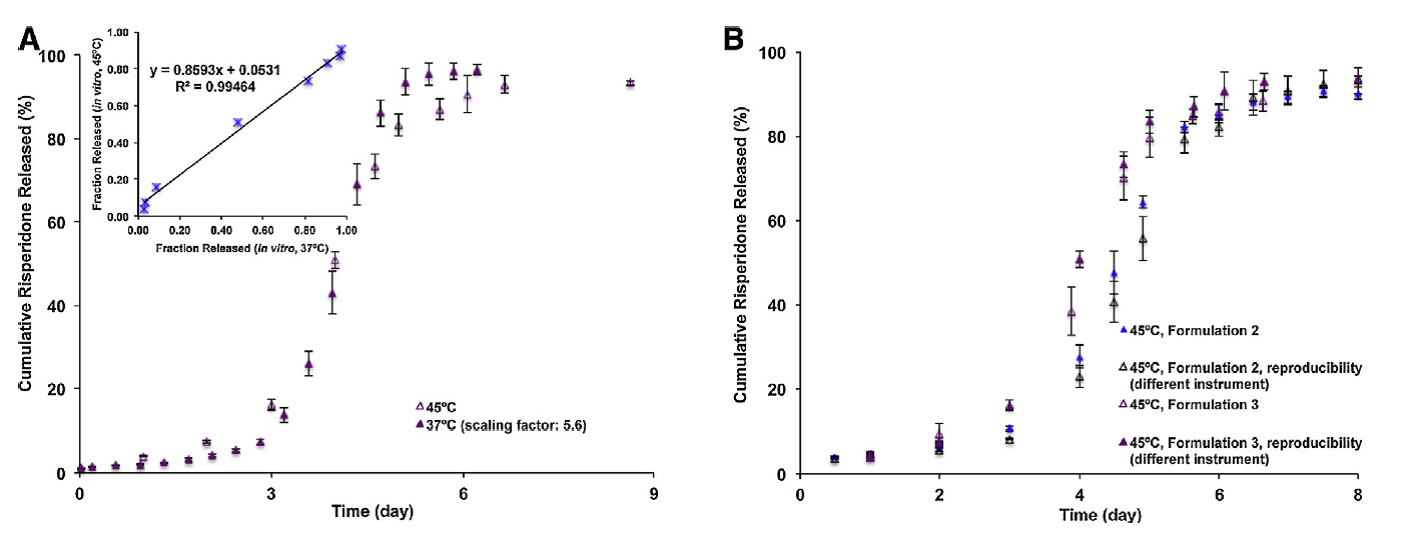
3) A reproducible accelerated in vitro release method using USP apparatus 4 for risperidone microspheres has been developed, which also correlates with the real time release kinetics as shown in Figure 3. This method can be adopted by pharmaceutical industries for quality control purpose of risperidone microspheres and the knowledge learnt will be helpful for developing accelerated in vitro release methods for other LAI formulations.
Figure 3 (A) In vitro release profiles of Formulation 3 in 10 mM PBS (pH 7.4) at 37 °C (time-scaled) and at 45 °C using the USP apparatus 4 method (n = 3). Insert figure shows the linear correlation between real-time (time-scaled) (37 °C) and accelerated (45 °C) fraction risperidone released. (B) In vitro release profiles of Formulations 2 and 3 in 10 mM PBS (pH 7.4) using the USP apparatus 4 method at 45 °C (n = 3) (Reference 3).
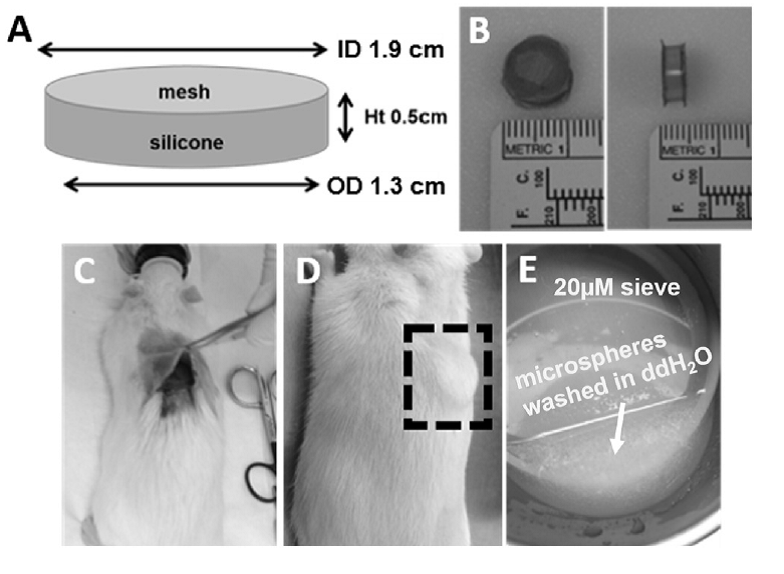
4) An unique cage model that allows retrieval of microspheres following administration has been developed as shown in Figure 4. This system will allow further analysis of particles after injection to obtain more mechanistic understanding of polymer degradation and drug release behaviors, thus facilitate development of IVIVCs of these drug products.
Figure 4. Cage implant design and administration in rats. (A) Schematic of cage design and dimensions. (B) Top view (left) and side view (right) of a cage implant. Cages were implanted in the subcutaneous space in rats (C and D). Following euthanasia, cages were retrieved and the microspheres were removed and rinsed on a sieve prior to analysis (E) (Reference 6).
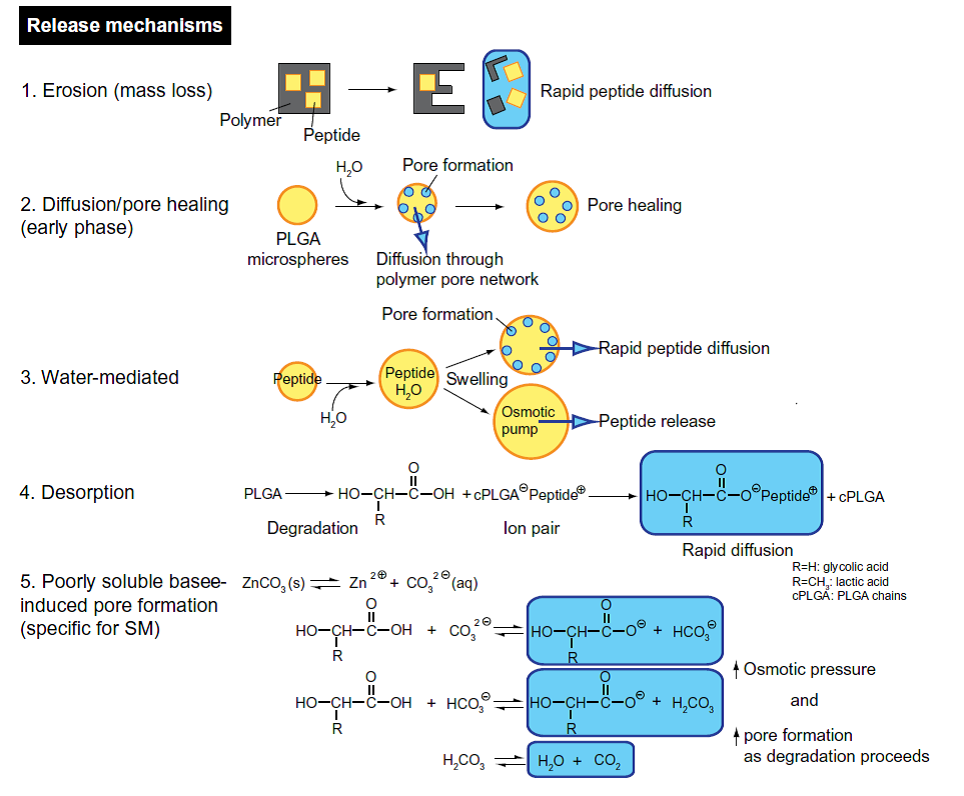
5) A mechanistic approach has been applied to understand release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development. Based on the in vitro evaluation, release mechanisms of leuprolide acetate are hypothesized as below (Figure 5). These information will provide better understanding of the release mechanisms of microsphere containing peptides.
Figure 5. Description of hypothesized release mechanisms of leuprolide-loaded PLGAmicrospheres (Reference 7).
6) New modeling and simulation tools to guide development of bioequivalence guidances for LAI microspheres are under progress. The developed tools will also be useful to facilitate optimizing design space for generic LAI formulation development.
ORS staff facilitating research in this area
- Stephanie Choi, Yan Wang, Yuan Zou
Projects and Collaborators
- In vitro-In vivo Correlations of Parenteral Microsphere Drug Products
- Site PI: Diane Burgess
- Grant #: 1U01FD004931-01
- Dissolution Methods for Parenteral Sustained Release Implant Drug Products
- Site PI: Diane Burgess
- Grant #: 1U01FD005169-01
- In vitro-In vivo correlations of parenteral microsphere drug products
- Site PI: Steven Schwendeman
- Grant #: 1U01FD005014-01
- Development of hydrogel-based in vitro dissolution apparatus for microparticle formulations
- Site PI: Kinam Park
- Grant #: 1U01FD005168-
- Influene of raw materials, manufacturing variables, and storage conditions on release performance of long-acting release microsphere products
- Site PI: Steven Schwendeman
- BAA #: HHSF223201510170C
- Computational drug delivery: leveraging predictive models to develop bioequivalent generic long-acting injections
- Site PI: Sam Rothstein
- BAA #: HHSF223201510102C
- Advanced analytical techniques for mixed polymer drug-delivery systems (New)
- Site PI: Haesun Park
- BAA #: HHSF223201610091C
- Investigation of peptide-polymer interactions in PLGA microspheres (New)
- Site PI: Steven Schwendeman
- Grant #: 1U01FD005847
Publications, Posters/Abstracts, and Presentations
- J. Garner, S. Skidmore, H. Park, K. Park, S. Choi, Y. Wang. A protocol for assay of poly(lactide-co-glycolide) in Clinical Products. (2015) International Journal of Pharmaceutics. 495(1)
- J. Shen, S. Choi, W. Qu,Y. Wang,D.J. Burgess. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. (2015) Journal of Controlled Release.
- J. Shen, K. Lee, S. Choi, W. Qu,Y. Wang, D.J. Burgess. A reproducible accelerated in vitro release testing method for PLGA microspheres. (2016) International Journal of Pharmaceutics. 498(1-2), pp. 274-282
- J. V. Andhariya, D.J. Burgess. Recent advances in testing of microsphere drug delivery systems. Expert Opinion on Drug Delivery. 2016, 13(4): 593-608.
- J. Shen, D.J. Burgess. In vitro-in vivo correlation for complex non-oral drug products: Where do we stand? Journal of Controlled Release. 2015, 219: 644-51.
- Doty, K. Hirota, K. Olsen, N. Sakamoto, Y.Wang, S. Choi, W. Qu, A. Schwendeman, S. Schwendeman. Validation of a cage implant system for assessing in vivo performance of long-acting release microspheres. (2016) Biomaterials.
- K. Hirota, A. Doty, R. Ackermann, J. Zhou, K.F. Olsen, M.R. Feng, Y. Wang, S. Choi, W. Qu, A.S. Schwendeman, S. Schwendeman. Characterizing release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: In vitro evaluation. (2016) Journal of Controlled release.
- J. V. Andhariya, J. Shen, S. Choi, Y. Wang, W. Qu, Y. Zou, D.J. Burgess. Research Highlight Talk in the session “Manufacture, Characterization, Stability, and Regulatory Aspect" at the CRS Annual Meeting & Exposition, WA, USA, July 2016.
- D.J. Burgess. "In Vitro-In Vivo Correlation for Complex Drug Products and In Vitro/In Vivo Stability Issues". Invited presentation, Peking University, Beijing, China, June 2016
- D.J. Burgess. "In Vitro-In Vivo Correlation for Complex Drug Products and In Vitro/In Vivo Stability Issues". Invited presentation, FDA, OGD, May 2016.
- J.V. Andhariya, J. Shen, S. Choi, Y. Wang, W. Qu, Y. Zou, D.J. Burgess. Effect of Manufacturing Processes on In Vitro and In Vivo performance of Naltrexone Microspheres, The American Association of Pharmaceutical Scientists Annual Meeting, Denver, CO, Nov. 2016.
- J. Shen, W. Qu, Y. Wang, S. Choi, D.J. Burgess. Effect of manufacturing process parameters on in vitro performance of peptide microspheres. The Controlled release meeting annual Meeting, Seattle, WA, USA, July 2016.
- J.V. Andhariya, N.K. Swarnakar, J. Shen, W. Qu, S. Choi, Y. Wang, D.J. Burgess. Effect of Manufacturing Processes on Critical Quality Attributes of Naltrexone Microspheres. The Controlled release meeting annual Meeting, Seattle, WA, July 2016.
- S.P. Schwendeman, K. Hirota, A.C. Doty, Y. Wang, S. Choi, W. Qu. Mechanistic evaluation of in vitro and in vivo release from PLGA microspheres employing a cage model. 14th European Symposium on Controlled Drug Delivery, Egmond ann Zee, The Netherlands, April 2016.
- K. Hirota, J. Zhou, R. Ackermann, Y. Wang, S. Choi, A. Schwendeman, S.P, Schwendeman. Reverse engineering of the one-month Lupron Depot®. The American Association of Pharmaceutical Scientists Annual Meeting, Denver, CO, Nov. 2016.
- J. Garner, S. Skidmore, H. Park, K. Park, S. Choi, Y. Wang. Assay of PLGA properties in Parenteral Depot Formulation. The Controlled release meeting annual Meeting, Seattle, WA, July 2016.
- J. Garner, S. Skidmore, H. Park, K. Park, S. Choi, Y. Wang. Assay of polymeric sustained systems for carrier properties. The American Association of Pharmaceutical Scientists Annual Meeting, Denver, CO, Nov. 2016.
Outcomes
- Revision of Product-Specific guidance on Risperidone Intramuscular Injection (Aug 2016)