Nucleus 24 Cochlear Implant System – P970051/S205
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Nucleus 24 Cochlear Implant System
PMA Applicant: Cochlear Americas
Address: 10350 Park Meadows Drive, Lone Tree, CO 80124
Approval Date: January 10, 2022
Approval Letter: Approval order
What is it?
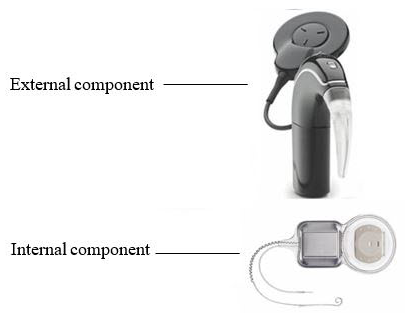
The Nucleus 24 Cochlear Implant System is an implant that gives a person access to sound by directly stimulating the hearing nerve (auditory nerve). The cochlear implant system consists of an internal and an external component. The Nucleus 24 Cochlear Implant System was previously approved for use in people ages 9 months and older with severe to profound sensorineural hearing loss in both ears. This approval expands the use for this device to include people ages 5 years and older with severe to profound hearing loss in one ear (single-sided deafness/unilateral hearing loss (SSD/UHL)) and normal hearing or mild hearing loss in the other ear.
How does it work?
The internal component of the Nucleus 24 Cochlear Implant System is implanted by a surgeon in the ear with SSD/UHL. A microphone in the external component picks up the sounds from the environment and turns it into digitally processed signals that are sent to the internal component that is implanted in the inner ear. The internal component sends electrical pulses to the cochlea in the inner ear after receiving the digitally processed signals from the external device. These electrical pulses travel along the auditory nerve to the brain where they are interpreted as sound.
When is it used?
The Nucleus 24 Cochlear Implant System is intended to help provide a sense of sound to people 5 years of age with SSD/UHL.
What will it accomplish?
The Nucleus 24 Cochlear Implant System may improve a person’s understanding of speech in noise, ability to identify the location or origin of a detected sound in direction (sound localization), and personal perception of their own hearing quality.
When should it not be used?
The Nucleus 24 Cochlear Implant System should not be used in people with:
- No cochlear development or no cochlear nerve
- Active middle ear infections
- A ruptured eardrum with active middle ear disease
- Profound sensorineural hearing loss for more than 10 years