Testimony
Event Title
The President's FY2022 Budget Request for FDA
June 10, 2021
- Testimony of
-
Janet Woodcock, M.D.
Acting Commissioner of Food and Drugs - Food and Drug Administration
Chair Baldwin, Ranking Member Hoeven, and Members of the Subcommittee, thank you for the opportunity to appear before you today to discuss the President’s Fiscal Year 2022 Budget (Budget) request for the Food and Drug Administration (FDA or the Agency).
I would like to begin by thanking the Subcommittee for your continued support of the Agency in recent years, and in particular the past year as the Agency has worked to address the current COVID-19 public health emergency. The funding increases that the Subcommittee provided to FDA have been essential to the Agency fulfilling its critical mission.
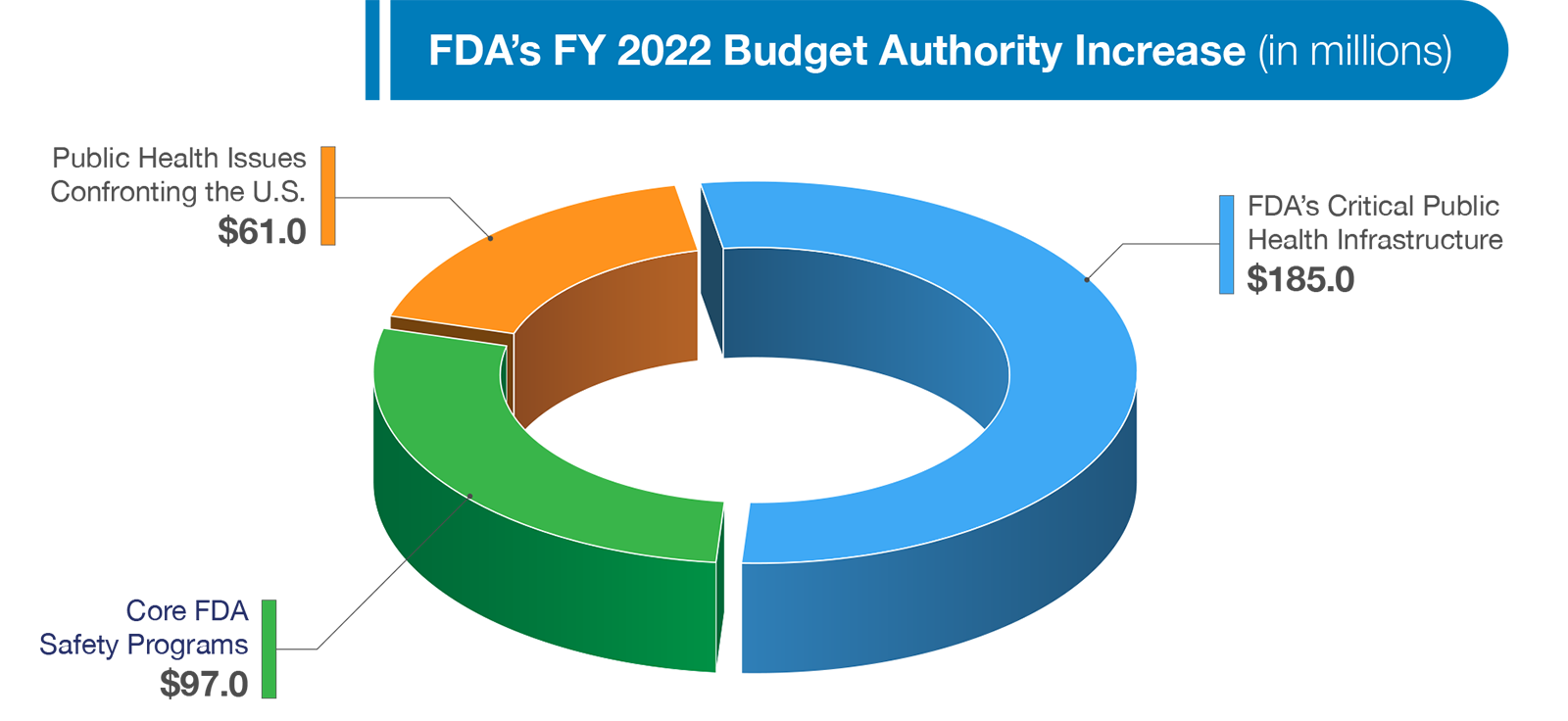
Today, I am pleased to present FDA’s FY 2022 Budget Request. Our program level request totals $6.5 billion, which is comprised of $3.6 billion in discretionary budget authority and $2.9 billion in user fees. This is an increase of eight percent, or $477 million above the FY 2021 Enacted level. The Budget requests a net budget authority increase of $322 million, which reflects $343 million in requested increases and scheduled adjustments of -$21 million to reflect the authorized level for 21st Century Cures and the one-time funding provided in FY 2021. The Budget focuses on necessary investments in three main categories: (1) critical public health infrastructure; (2) core FDA safety programs; and, (3) public health issues confronting the nation today.
I. Critical Public Health Infrastructure
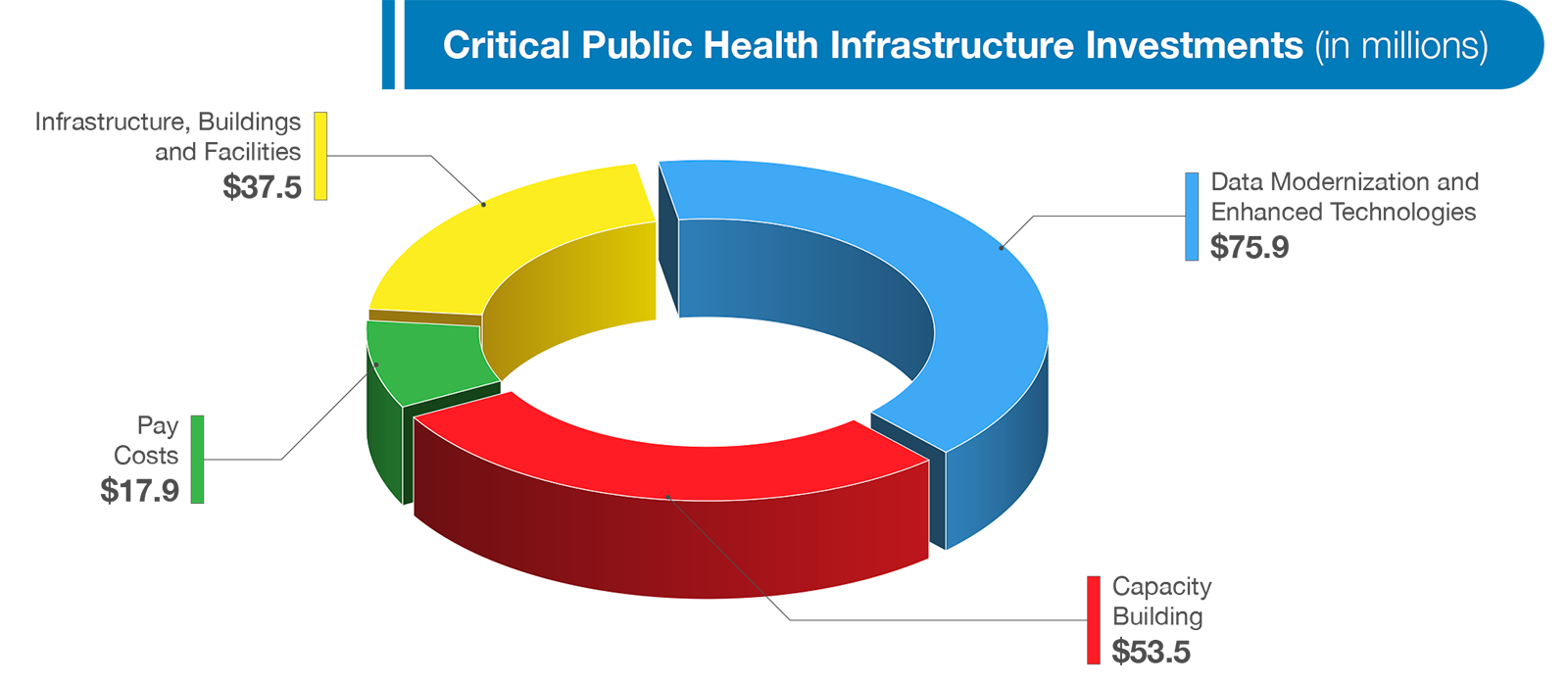
FDA’s Budget requests an increase of $185 million to support our critical public health infrastructure. This infrastructure is fundamental to every aspect of our work. Challenges faced during the current pandemic have reinforced the need to ensure that the Agency is making consistent, smart investments with respect to our physical and technological infrastructure so that we are prepared to effectively fulfill our core mission and tackle unpredictable public health crises.
With that in mind, the Agency’s infrastructure request includes critically important investments in data modernization, maintenance and repairs to our facilities, expanded laboratory safety efforts, and an increase in internal capacity to support our workforce of over 18,000, including targeted investments in the Office of Chief Counsel, cybersecurity, IT equipment replacement, and business functions within the Office of Operations. A few of these investments requested in this year’s Budget are highlighted in more detail below.
Data Modernization
The Budget requests an increase of $75.9 million to support FDA data modernization efforts. As witnessed firsthand during the COVID-19 pandemic, technology has and will continue to revolutionize human and animal health. Scientific breakthroughs have enabled the development of new, more personalized therapeutic treatments, advanced manufacturing, and state-of-the-art solutions such as blockchain, genomic information, and real-time analytics. As a byproduct, the amount and variety of data that FDA generates, needs, and uses is rapidly increasing at exponential rates. However, the Agency utilizes antiquated methods, including inspecting large volumes of PDFs, often “by hand,” in order to identify critical safety signals, such as human and animal drug and device safety concerns or emerging foodborne outbreaks.
In response, the Budget requests investments to modernize the Agency’s data infrastructure. The request includes two components—an Agency-wide component ($44.5 million) and complementary program-specific investments ($31.4 million). Combined, this funding will allow FDA to more efficiently gather data; identify, analyze and respond to potential problems more quickly; and, to further improve review times for medical products.
Buildings and Facilities Infrastructure
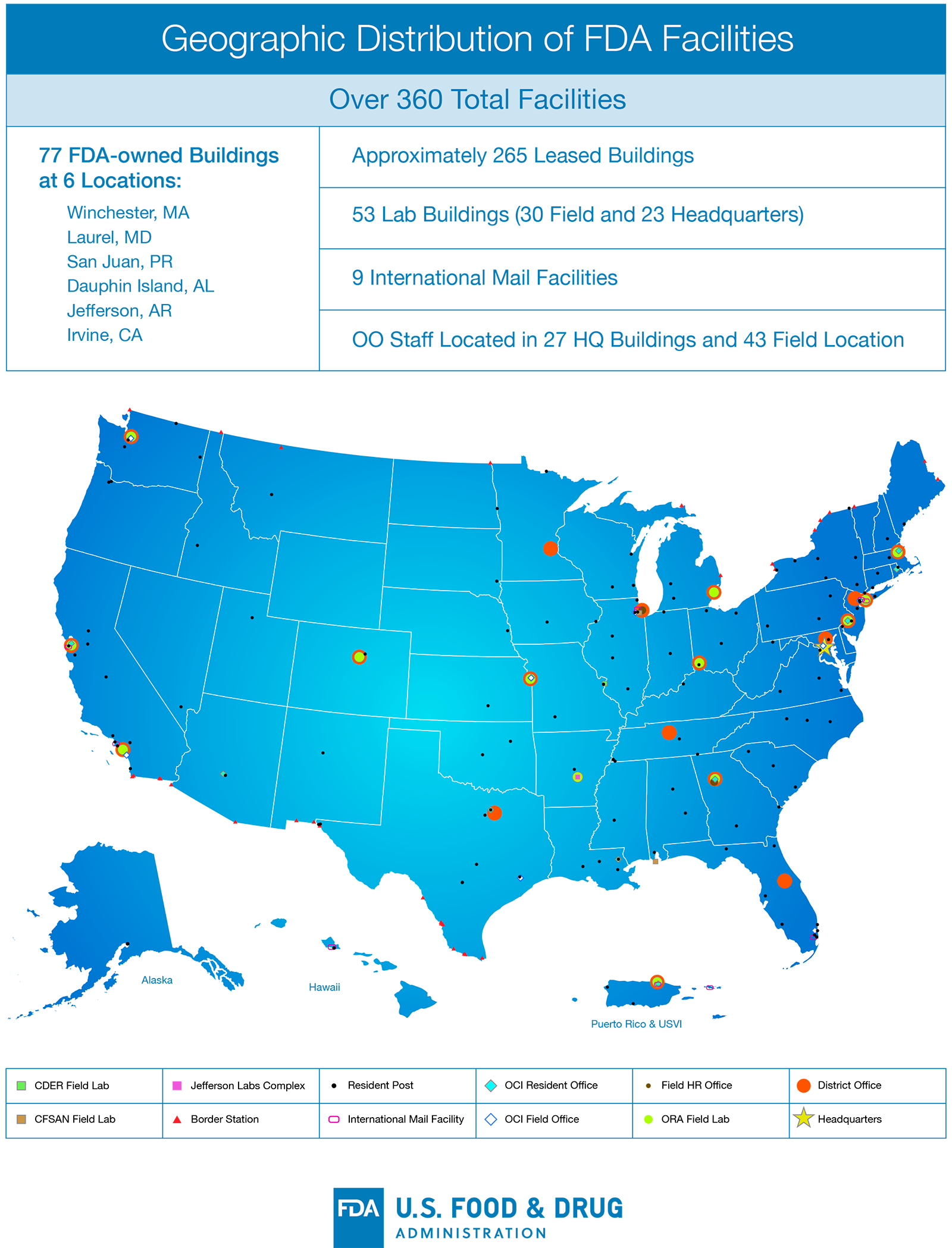
The Budget requests an increase of $37.5 million for FDA’s owned and leased facilities’ needs. FDA’s facilities requirements during any given year are diverse and cover 77 FDA-owned buildings at six locations across the U.S. and Puerto Rico and approximately 265 leased buildings, including 53 laboratories. Repairs and upgrades needed at these facilities vary from basic maintenance to complex laboratory retrofits. This requested increase will allow FDA to better operate, maintain, and secure its owned and leased facilities, as well as make necessary repairs and improvements at both the FDA White Oak Campus and FDA owned buildings across the country. FDA’s current backlog of maintenance and repairs at our owned locations is greater than $220 million and places a considerable strain on the Agency’s ability to execute its mission. Maximizing the public health value of FDA funding is paramount and we will continue to prioritize our facility investments to ensure that our owned and FDA-occupied facilities are efficient, cost-effective and meet the demands of FDA’s expanding workload and workforce.
II. Core FDA Safety Programs
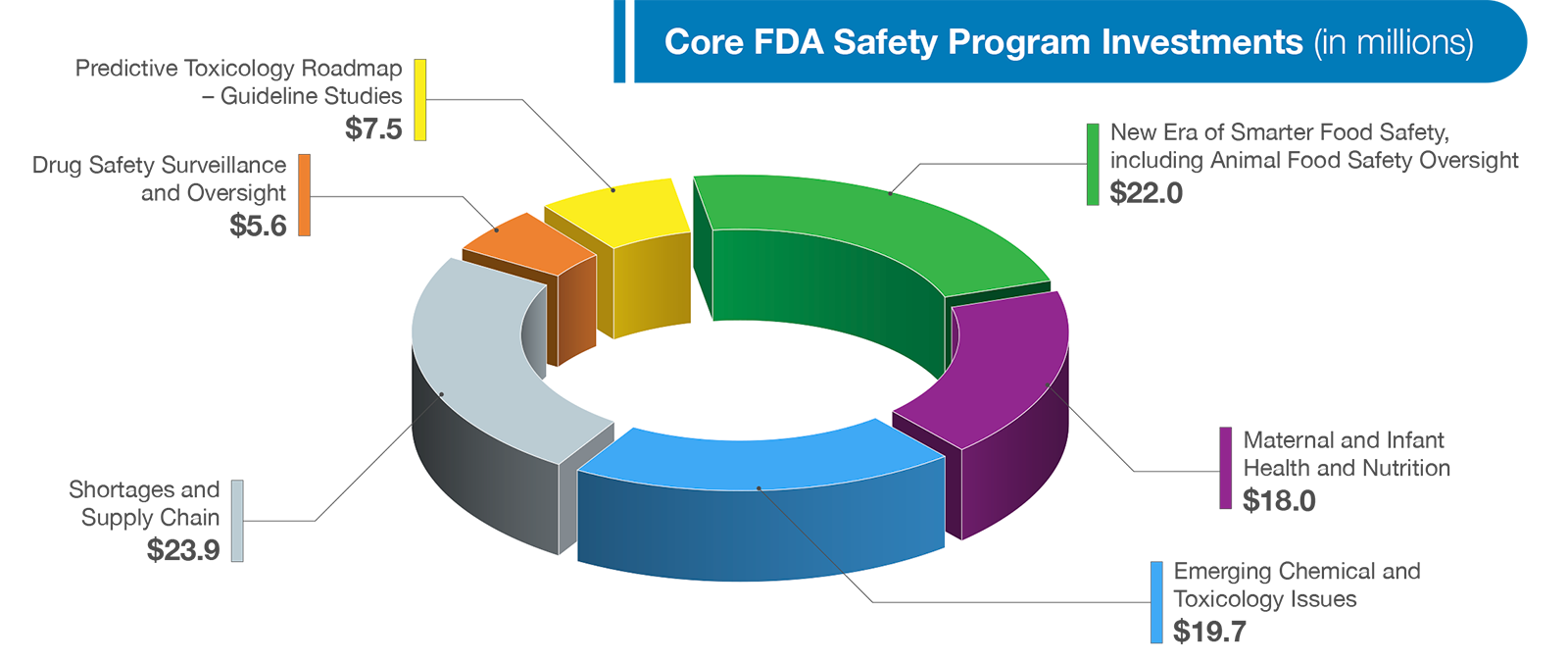
FDA’s responsibilities continue to expand and grow in complexity due to advances in food and medical product technology, globalization of supply chains, increasingly complex and diverse data sources, and emerging scientific approaches. The nation relies on FDA to provide rigorous and transparent scientific review, a predictable and responsive regulatory structure, comprehensive inspections at domestic and foreign food and medical product manufacturers, and expert staff to provide support for these activities. The Budget requests an increase of $97 million to invest in core food and medical product safety programs to reduce the number of foodborne illnesses through implementation of the New Era of Smarter Food Safety Blueprint; improve maternal and infant nutrition and health; address emerging food-related chemical and toxicological issues; strengthen oversight of animal foods, strengthen and monitor the device supply chain; and increase drug safety surveillance and oversight. A few of the investments requested in this year’s Budget are highlighted in more detail below.
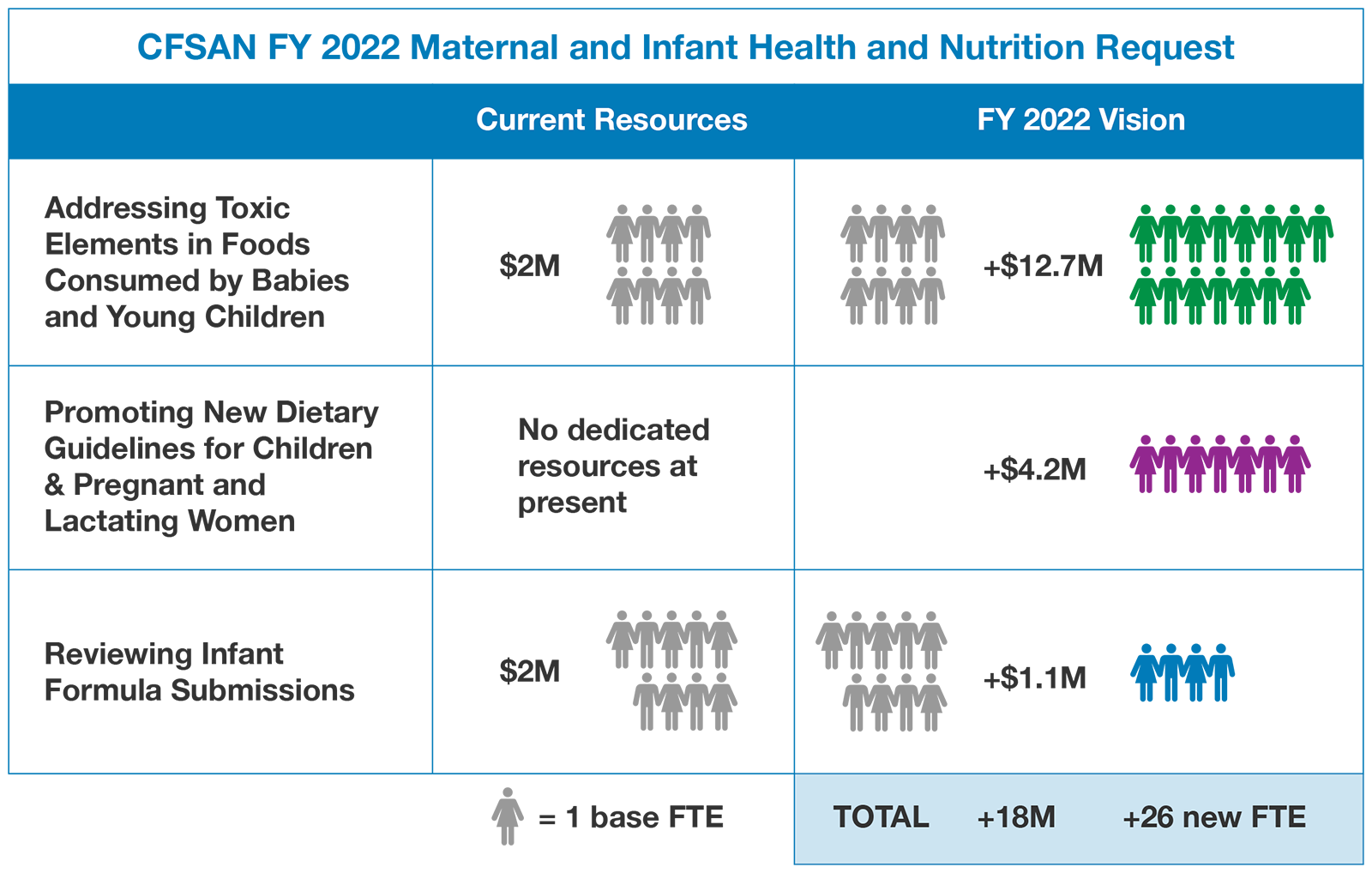
Maternal and Infant Nutrition and Health Initiative
Nutrition during pregnancy and in early childhood is critically important in supporting the health and wellbeing of mothers and their children and key to promoting health equity. Nutrients vital for brain development and growth must be provided in adequate amounts during early years, whereas certain dietary constituents should be limited, and some should be avoided altogether to prevent potentially irreversible harm. Infants, young children, and the developing fetus are especially vulnerable to toxic elements (e.g., lead, cadmium, arsenic, and mercury) due to small body size and rapid growth and development.
With its available regulatory tools and authorities, the FDA is uniquely positioned to advance work that promotes healthy dietary patterns and consumption of essential nutrients for pregnant women, infants and young children, while mitigating risk of dietary exposure to toxic elements harmful for growth and development in early life. As such, the Budget requests $18 million to support work that advances both nutrition and food safety at the Center for Food Safety and Applied Nutrition (CFSAN). The funding will support three components: (1) Enhancing Infant Formula Premarket Review; (2) Addressing Toxic Elements in foods commonly consumed by babies and young children; and (3) Promoting Dietary Patterns Recommended by the Dietary Guidelines while Mitigating Risk of Exposure to Dietary Toxicants. This funding will expand the Agency’s capacity to review the increasing number, size, and complexity of infant formula submissions and will push to reduce exposure to toxic elements from foods eaten by babies and young children to as low as possible.
Medical Product Supply Chain
The COVID-19 pandemic has exposed great weaknesses in the medical product supply chain’s dependence on foreign medical products. To ensure the U.S. is properly prepared now, and in the future, we must take action to secure our medical product supply chain, including related ingredients and components.
The Budget requests an increase of $21.6 million for a new Resilient Supply Chain and Shortages Program at the Center for Devices and Radiological Health (CDRH). This funding will provide resources to establish a permanent program for U.S. supply chain resilience for medical devices for the first time. The funding will help to stand up this program that will focus on strengthening the domestic supply chain through investments in preventive measures, identifying potential medical product supply short-falls, continuing surveillance, and rapid intervention.
FDA is also requesting an additional $2.3 million for medical product supply chain efforts by the Center for Veterinary Medicine (CVM) to strengthen its data analytics capacity to help identify and anticipate the effects of the public health emergencies on the animal drug supply. Finally, FDA is requesting an increase of $5.6 million for Drug Safety Surveillance and Oversight activities for the Center for Drug Evaluation and Research (CDER).
III. Public Health Issues Confronting the United States
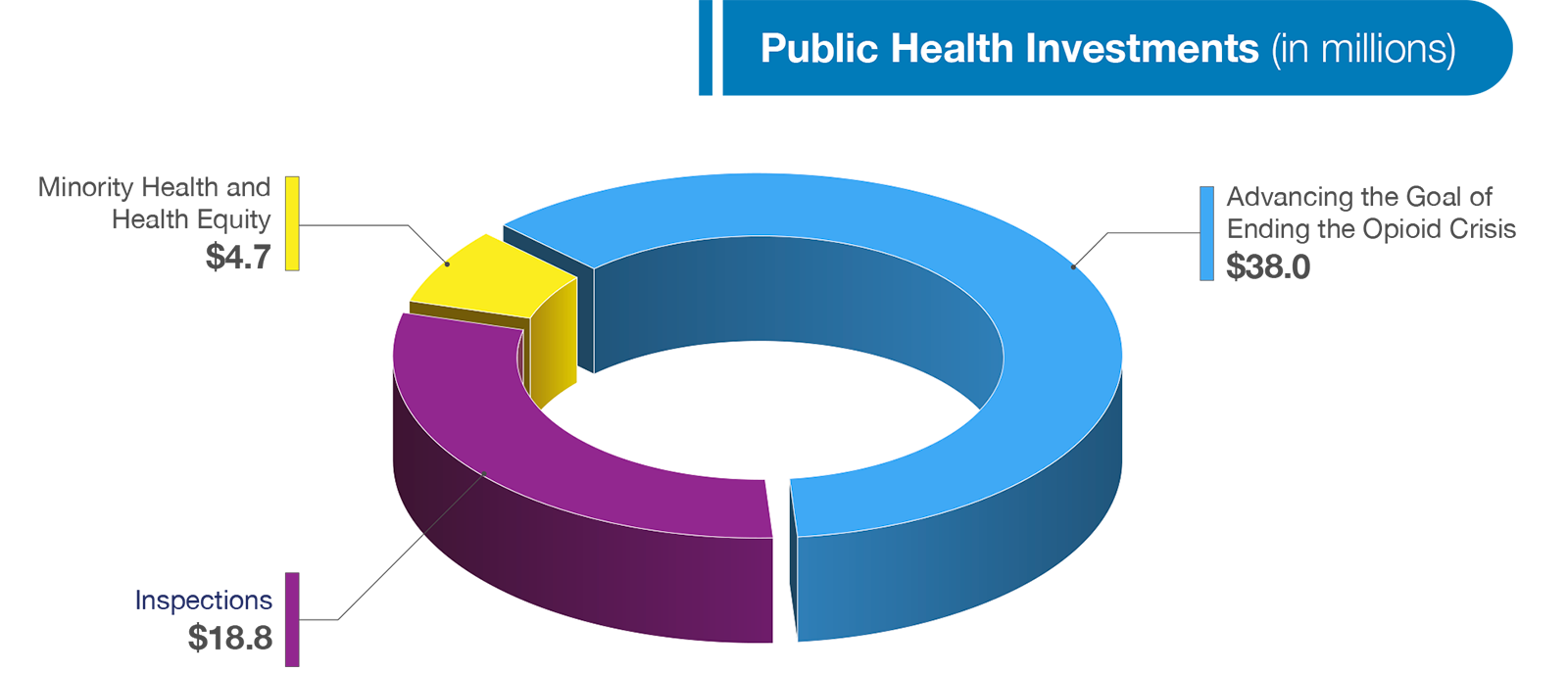
In addition to strengthening our fundamental infrastructure and supporting our core medical product and food safety programs, in the upcoming year we must also focus on the most pressing public health issues facing our country. While the COVID-19 pandemic has dominated headlines over the past year, other longstanding health challenges have surged back into the spotlight and our Budget requests an additional $61 million to further our efforts to address these public health challenges. Funding will support several activities, including, efforts to increase safe and secure inspections, promote health equity, and address the opioid crisis. A few of these investments requested in this year’s Budget are highlighted below in more detail below.
Safe and Secure Inspections
FDA’s inspectional activities, coordinated by the Office of Regulatory Affairs (ORA), play a crucial role in the mission of the Agency to protect consumers and enhance public health by ensuring access to FDA regulated products and minimizing risk associated with those products. ORA inspects regulated products and manufacturers, conducts sample analyses of regulated products and reviews imported products offered for entry into the United States. To support our critical inspection work in the upcoming fiscal year, our Budget requests an increase of $18.8 million to ORA’s base funding to support for our inspections program.
With support from COVID-19 supplemental funding, ORA is working on COVID-19 recovery activities including inspectional modernization, preparing to adjust staffing and onboarding new staff to support onsite inspections. The resources requested as part of the FY 2022 Budget will allow ORA to maintain the staff hired with supplemental funding and increase our foreign inspection teams. We are also actively working to expand upon the use of remote assessment tools where appropriate, such as remote livestreaming video of operations, teleconferences, or screen sharing. These remote assessments, used in combination with other tools, enable the FDA’s investigators to assess the level of compliance and process control at sites.
Expand health equity and health disparity efforts
I am pleased to request an increase of $4.7 million to enhance FDA’s ability to support and expand health equity and health disparity efforts. The COVID-19 pandemic is only the latest example of the disproportionate impact public health problems have on minority and underserved communities. This funding will allow FDA to expand culturally and linguistically tailored communication and outreach efforts, establish new scientific initiatives, support novel health disparity and health equity focused intramural and extramural research, advance activities that enhance meaningful inclusion of minority populations in clinical trials, and understand and address health disparities. In addition, the funding will allow FDA to increase engagement with Historically Black Colleges and Universities, Minority Serving Institutions, and other collaborators to address gaps and needs of underserved communities, and develop FDA-wide training programs that focus on the reduction of health disparities and advancement of health equity.
Ending the Opioid Crisis
FDA will continue to address the opioid crisis that has only been exacerbated by the COVID-19 pandemic. As part of HHS’s Department-wide initiative to Advance the Goal of Ending the Opioids Crisis, in FY 2022 FDA requests an increase of $38 million to support activities in CDER, ORA, and CDRH. As part of the initiative, CDER will receive $26 million to support development of opioid overdose reversal treatments and treatments for Opioid Use Disorder (OUD). CDER will, among other activities advance the development and adoption of evidence-based clinical practice guidelines for acute pain conditions; assess feasibility to integrate opioid Risk Evaluation and Mitigation Strategies (REMS) education into IT health systems/Electronic Health Records and explore use of health IT systems to support goals of REMS, such as prescriber education; and continue to support opioid research efforts. Within CDRH, $2 million will be invested in efforts that will allow FDA to advance the development, evaluation, and market authorization of digital health medical devices that help address OUD.
Finally, of the $38 million increase, the Budget will provide ORA $10 million to establish satellite laboratories at the Agency’s International Mail Facilities (IMFs). FDA staff are assigned to nine IMFs throughout the U.S., Puerto Rico and the U.S. Virgin Islands and are responsible for monitoring mail importations of FDA regulated products by conducting comprehensive examinations of packages suspected of containing drugs to determine if those drugs should be refused delivery to the U.S. consumer. The Budget request will also fund permanent staffing at the IMFs by scientists along with expanding ORA’s use of analytical tools for screening entries, expand the current IMF initiative to interdict shipments of opioids, unapproved foreign drugs, counterfeit pharmaceuticals and health fraud related shipments, and support Pharmacy Compounding and Outsourcing Facility inspections, which include an inspectional assessment for compounding or repackaging of opioid products.
IV. Conclusion
I would like to close by thanking the Subcommittee again for your continued support of the Agency. As the gold standard for protecting and promoting public health, FDA is trusted by Americans and admired around the world for our work ensuring the safety, efficacy, and security of our nation’s medical products and the safety of our food supply. Once again, thank you for inviting me. I look forward to answering your questions.