Neonatology
Provides information on neonatology considerations raised in the development and use of FDA-regulated products in newborns
Our mission:
To lead the effort to deepen and broaden the therapeutic toolbox for neonatal conditions, with an emphasis on safety, efficacy, and labeling.
What we do:
- Provide neonatal-perinatal medicine consultations and expertise upon request to any center or office within the FDA related to any stage of neonatal or perinatal product development
- Provide presentations at internal and external events by invitation; train FDA staff on neonatology considerations in FDA-regulated product development
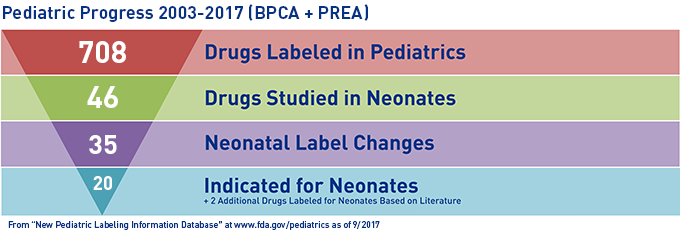
- Explore neonatology considerations raised by pediatric studies required under the Pediatric Research Equity Act or requested under the Best Pharmaceuticals for Children using OPT’s custom databases
International Neonatal Consortium (INC)--])
The Neonatology Team participates in the International Neonatal Consortium (INC), a global collaboration formed to forge a predictable regulatory path for evaluating the safety and effectiveness of therapies for neonates.
Regulations and Guidances
Food and Drug Administration Safety and Innovation Act (FDASIA) - Title V- Pediatric Drugs and Devices
Guidance for Industry
- The Content and Format for Pediatric Use Supplements
- Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Pediatric Study Plans
- General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products
Selected publications
1. Enrollment of Neonates in More Than One Clinical Tria![]() l.
l.
Jonathan M. Davis MD, Gerri R. Baer MD, Ronald Portman MD, Robert Nelson MD, PhD, Linda Storari, Jacob V. Aranda MD, PhD, Ralph Bax MD, Anne Zajicek MD, PharmD, Agnes Klein MD, DPH, Mark Turner MD, Simin Baygani, Merran Thomson MD, Karel Allegaert MD for the International Neonatal Consortium
Clinical Therapeutics. DOI: 10.1016/j.clinthera.2017.09.006 | First published October 4, 2017
2. Neonatal Safety Information Reported in the FDA During Drug Development Studies![]() .
.
Debbie Avant, R.Ph., Gerri Baer, M.D., Jason Moore, Pharm.D, Panli Zheng, B.S., Alfred Sorbello, D.O., MPH, Ron Ariagno, M.D., Lynne Yao, M.D., Gilbert J. Burckart, Pharm.D., Jian Wang, Ph.D.
Therapeutic Innovation and Regulatory Science. DOI : 10.1177/2168479017716713 | First Published June 28, 2017
3. Pharmacology Review: The Role of Biomarkers and Surrogate End Points in Drug Development for Neonatal Pulmonary Arterial Hypertension![]()
Haihao Sun, MD, PhD; Norman Stockbridge, MD; Ronald L. Ariagno, MD; Dianne Murphy, MD
NeoReviews. Feb 2016, 17 (2) e87-e92; DOI: 10.1542/neo.17-2-e87
4. A survey of neonatal pharmacokinetic and pharmacodynamic studies in pediatric drug development.
Jian Wang, PhD, Debbie Avant, RPh, Dionna Green, MD, Shirley Seo, PhD, Jeffrey Fisher, PhD, Andrew E. Mulberg, MD, Susan K. McCune, MD, Gilbert J. Burckart, PharmD
Clin Pharmacol Ther. 2015 May 14. doi: 10.1002/cpt.149
5. Predicting Neonatal Pharmacokinetics from Prior Data Using Population Pharmacokinetic Modeling.![]()
Jian Wang, PhD, Andrea N. Edginton, PhD, Debbie Avant, RPh, Gilbert J. Burckart, PharmD
J Clin Pharmacol, April 19, 2015. DOI 10.1002/jcph.524
6. Ethical considerations in conducting pediatric and neonatal research in clinical pharmacology.
Roth-Cline M, Nelson RM.
Curr Pharm Des. 2015; 21(39): 5619-35
7.Drug Labeling and Exposure in Neonates. ![]()
Matthew M. Laughon, MD, Debbie Avant, RPh, Nidhi Tripathi, MD, Christoph Hornik, MD, Michael Cohen-Wolkowiez, MD, Reese Clark, MD, P. Brian Smith, MD, William Rodriguez, MD, PhD
JAMA Pediatr. 2014 Feb;168(2):130-6