Pediatric Safety
Medication use in the United States has changed substantially since requirements for drug efficacy were enacted by Congress in 1962 1. Today, millions of people depend on medications to sustain their health.
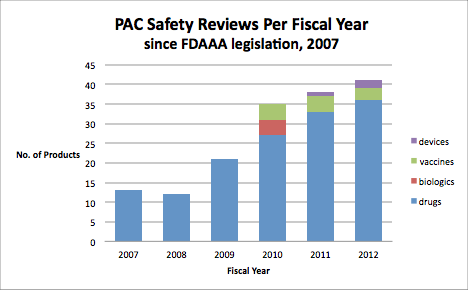
Post-marketing safety reviews are particularly important for pediatrics. Children are a smaller percentage of the population and tend to be healthy. Therefore, pediatric product development studies, other than for vaccines or some childhood illnesses such as otitis media, tend to be small due to the limited number of children available for clinical trial participation. However, once a product is approved for either adults or older children, it is frequently used off-label for other ages and indications. Because of the limited number of children in studies, the post-market pediatric experience is crucial to understand safe use in the pediatric population. Drug use in the pediatric population continues to rise due to improved diagnostics, therapies, and off-label use 2. The Congressionally mandated pediatric safety reviews presented to the Pediatric Advisory Committee (PAC) enhance further our understanding of the safety of a product and the risk/benefit of products used in children.
The Safety Team
The Office of Pediatric Therapeutics Safety Team implements the mandated pediatric-focused safety reviews of drugs, biologics, vaccines, and pediatric Humanitarian Device Exemption devices studied in the pediatric population. Every year this Office convenes 2-3 PAC meetings to obtain advice on the safety assessments for these products. The Safety Team is responsible for coordinating all the scientific reviews related to this safety process within FDA and managing the presentations at the meetings. In addition to the PAC, the Safety Team is directly involved with a pediatric product safety initiative utilizing FDA’s existing MedSun/KidNet network to address queries from the PAC and is tailored to study pediatric drug use and safety concerns in the neonatal settings.
- The 1962 Kefauver-Harris Drug Amendments, for the first time, required drug manufacturers to prove to FDA the effectiveness of their products before marketing them.
- NEJM 347(18):2002;1462-70
Important Links
- FDA Pediatric Safety Communications
- Safety Reporting: Special Post-Marketing Reviews
- New Pediatric Labeling Information Database
- Premarket Assessment of Pediatric Medical Devices
- Consumer Updates: Children's Health
Product Recalls
FDA Notifications and Alerts
MedSun
- MedSun KidNet Roundtable Discussion (2008)
- MedSun KidNet Roundtable Discussion (2007)
- MedSun: Medical Product Safety Network