Pediatric Science and Research Activities
The Office of Pediatric Therapeutics (OPT) is engaged in Pediatric Science and Research Activities. The objective of these activities is directed towards analysis of submitted pediatric trials, identified ethical and safety issues, and publication and dissemination of data and results.
The Pediatric Labeling Database highlights key pediatric information in labeling from the studies submitted in response to pediatric legislative initiatives. You can search for information by various methods including by the product’s trade name, generic name, or by the condition for which it was studied.
Pediatric Clinical Trials Database
The Pediatric Clinical Trials Database summarizes pediatric clinical trials submitted in response to pediatric legislative initiatives that resulted in new pediatric labeling.
Extrapolation in pediatric drug development
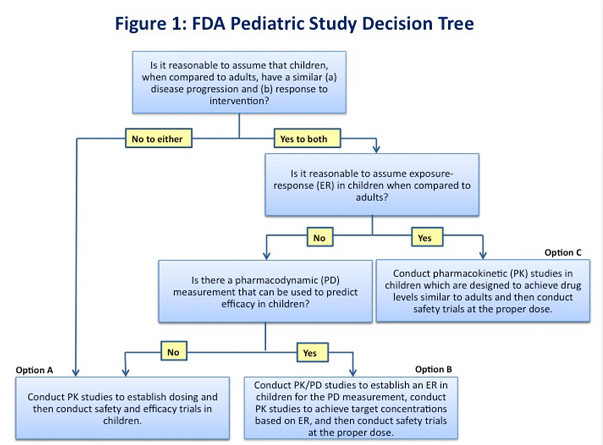
New drug development should be as efficient as possible, particularly in pediatric drug development when the available patient population is relatively small and has special vulnerabilities. The extrapolation of efficacy from adult and other data to the pediatric population minimizes the exposure of children to clinical trials, increases the speed and efficiency of pediatric drug development and allows pediatric patients timely access to safe and effective medicines. The FDA Pediatric Study Decision Tree (Figure 1) provides a simple assumptions-based framework that can be a helpful starting point in determining the pediatric studies (excluding oncology studies) necessary for labeling based on the ability to extrapolate efficacy from adult or other data.
If the assumptions required for extrapolation do not apply (Option A, Figure 1), then extrapolation cannot be used and efficacy must be demonstrated independently in the pediatric population by conducting two adequate and well-controlled trials. Pediatric pharmacokinetic (PK) studies should be conducted by using adult PK data to establish the correct dose for the condition or disease of interest. In some cases, pharmacokinetic modeling may be helpful to select the range of doses to be used in the studies. A crucial factor in designing pediatric efficacy studies is that the clinical endpoints must be age and developmentally appropriate and validated for use in the pediatric age group under study. For some indications, reliable Patient Reported Outcomes (PROs) have not been developed. The lack of consistent, validated PRO tools that are qualified for regulatory use has led to inefficiencies in drug development and in FDA’s review processes. Also, this may result in inconsistencies in the types of outcome information contained in the labeling for drugs approved for the same indication. The top 8 areas where PROs are needed are Asthma, Depression, Diabetes, Irritable Bowel Syndrome (IBS), Oncology, Pain, Cognition and Fatigue.
A different approach is used in pediatric oncology studies. In children, the efficacy cannot be extrapolated because pediatric tumors are rare and biologically distinct from tumors in adults. The disease specific surrogates or clinically relevant endpoints, and an evidence of a tumor response in the early phase studies are required to continue pediatric drug development.
More information on extrapolation can be found at the following OPT publication:
Dunne J, Rodriguez WJ, Murphy MD et al. Extrapolating efficacy: maximizing the use of adult and other data in pediatric drug development programs. ![]()
Neonatal Studies- Under Assessment
The Neonatal Studies Database summarizes clinical trials that include neonates submitted in response to new pediatric legislative initiatives that resulted in new pediatric labeling.
Kidnet Initiative
The Pediatric Drugs KidNet Initiative is an ongoing active surveillance program that interfaces directly between FDA and healthcare professionals. The program provides a better understanding of pediatric drug use and timely adverse event reporting from clinical sites across the country. The data is collected from Neonatal Intensive Care and Pediatric Intensive Care Units that are participating hospitals in the Center for Devices and Radiological Health’s (CDRH) MedSun program. This program will provide important pediatric safety information, such as the identification of emerging drug safety issues to help focus public health measures, strategies and interventions.
Econometrics
OPT creates and analyzes datasets derived from clinical trials as a result of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA).We are currently examining if econometric regression can predict which types of drugs are more or less likely to be found efficacious in children. Analyses include annual drug sales data, sponsor’s trial data, and FDA decisions on granted exclusivity in the case of BPCA.
Globalization
The U.S. Food and Drug Administration receive data generated from pediatric clinical trials conducted globally. In 2012, OPT reviewed 346 pediatric trials on drugs and biological products provided to FDA in submissions that were approved between 9/28/2007 and 12/21/2010. For each trial, the age range, therapeutic indication, design, duration, and patient and center enrollment by location were extracted and the results were compared with data from 2002-2007 (see Globalization Facilitates Pediatric Drug Development in the 21st Century. Julia Dunne, Lala Margaryants, M. Dianne Murphy, Ann M. Myers, Debbie Avant and William J. Rodriguez Drug Information Journal 2010 : 44(06)). A new study published in 11/2012 showed that the U.S. participated in 86% of a total of 346 studies and remains an important location for pediatric trials.