How to Enter a Certificate of a Pharmaceutical Product (CPP) Application
How to Enter a Certificate of a Pharmaceutical Product (CPP) Application Step-by-Step Instructions
July, 2014
Table of Contents
- Enter a Certificate of a Pharmaceutical Product (CPP) Application
- Navigation
- Section 1 Requestor and Billing Information
- Section 2
- Section 1.0 Proprietary name and Dosage form
- Section 1.1 Active ingredient and Amount per unit dose
- Section 3: Section 2A.1 & 2A.2 Applicant Address and Marketing Status Number
- Section 4: Section 2A.3 Status of the Product license holder
- Section 5
- Section 2B.2 Facility Information
- Section 2B.2 Add Remarks
- Section 6 Importing Countries
- Section 7 Number of Certificates Requested
- Section 8 Exporter’s Certification Statement (ECS)
- Final Review Page
Enter a Certificate of a Pharmaceutical Product (CPP) Application
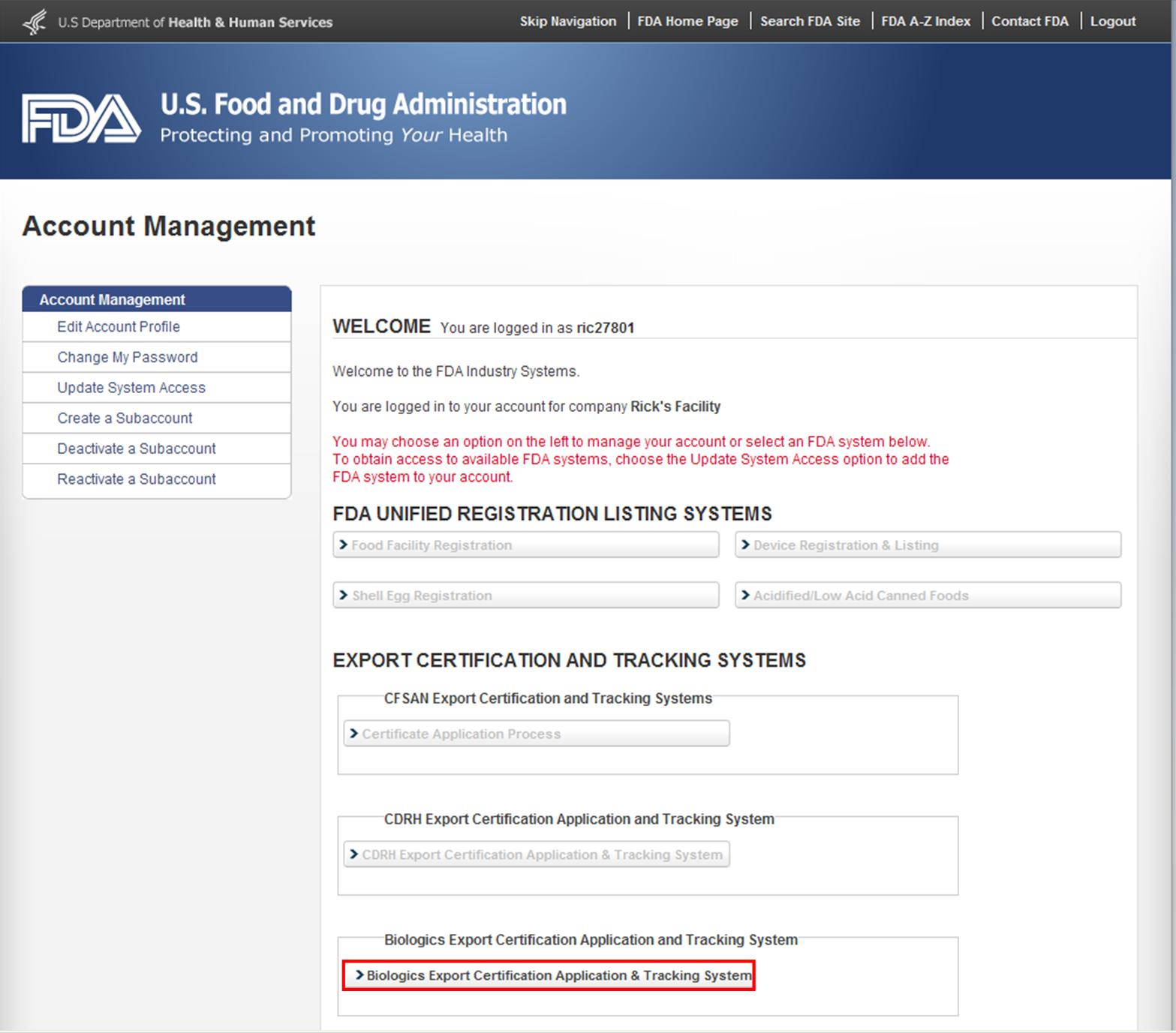
After you have logged into the FDA Industry Systems, select ‘Biologics Export Certification Application & Tracking System’ (BECATS) from the list of systems available on the FURLS Home Page as shown in Figure 1 below.
Figure 1: FDA Industry Systems Page
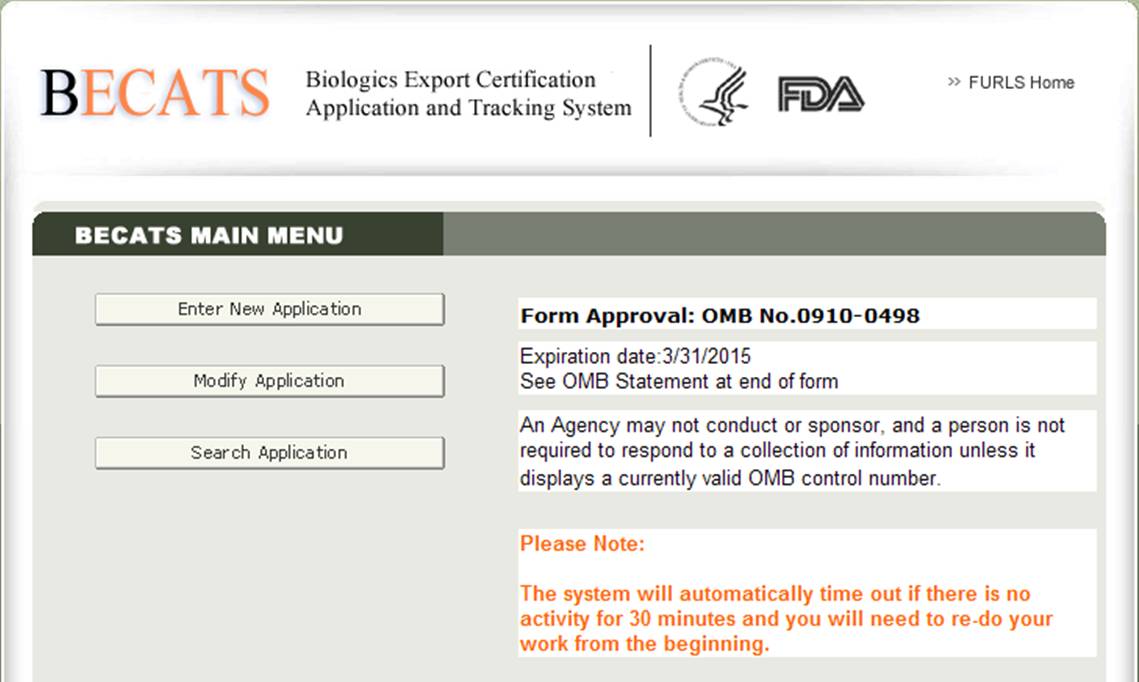
Once you have selected ‘Biologics Export Certification Application & Tracking System’, you will navigate to the BECATS Main Menu page as shown in Figure 2 below.
Figure 2: BECATS Main Menu
To begin the application process, select "Enter New Application" from the list of options. You may select "Modify Application" (when applicable) or "Search Application" for an existing application from the main menu.
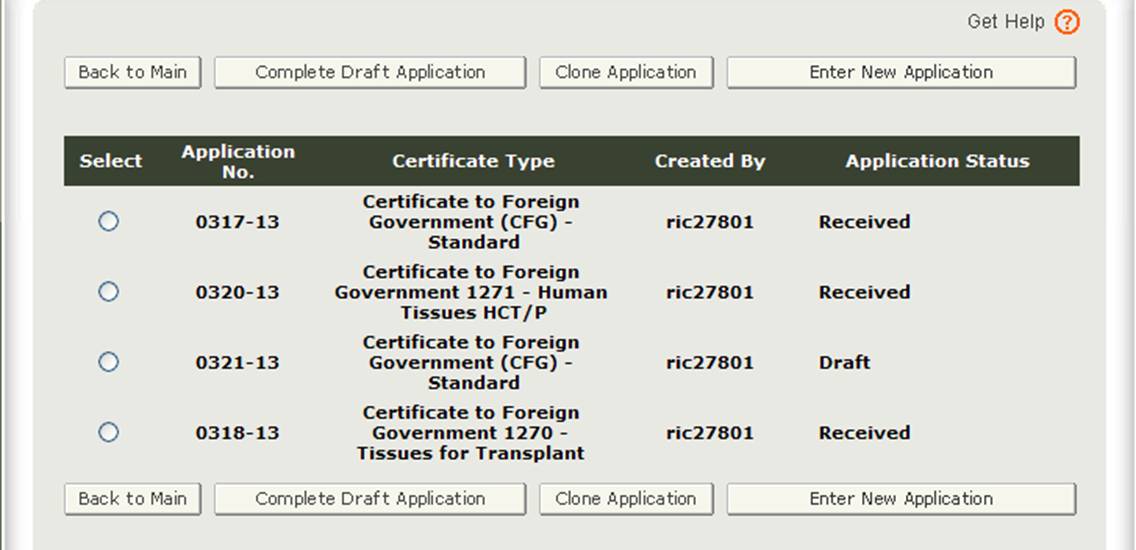
After you select the "Enter New Application" option, the system will display all applications that you have saved or submitted as shown in Figure 3 below.
Figure 3: Account Applications
Applications that are saved but not submitted will be in "Draft" status until you submit them.
- If you wish to continue working on an application that has been saved, select the desired application radio button and click on "Complete Draft Application".
- If you wish to copy an existing application, select the desired application radio button and click on "Clone Application". Please refer to "Create an application based on the existing application" section under the Modify Application of this document for more details.
- If you wish to create a new application, click on "Enter New Application".
Click on "Enter New Application" to create a new application.
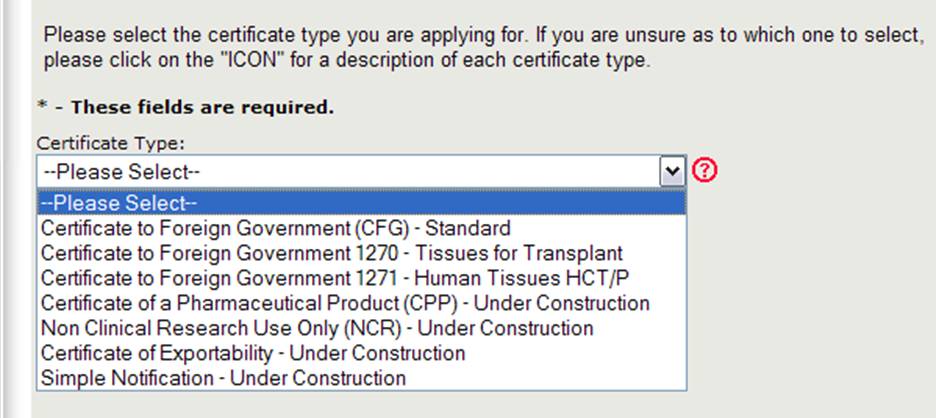
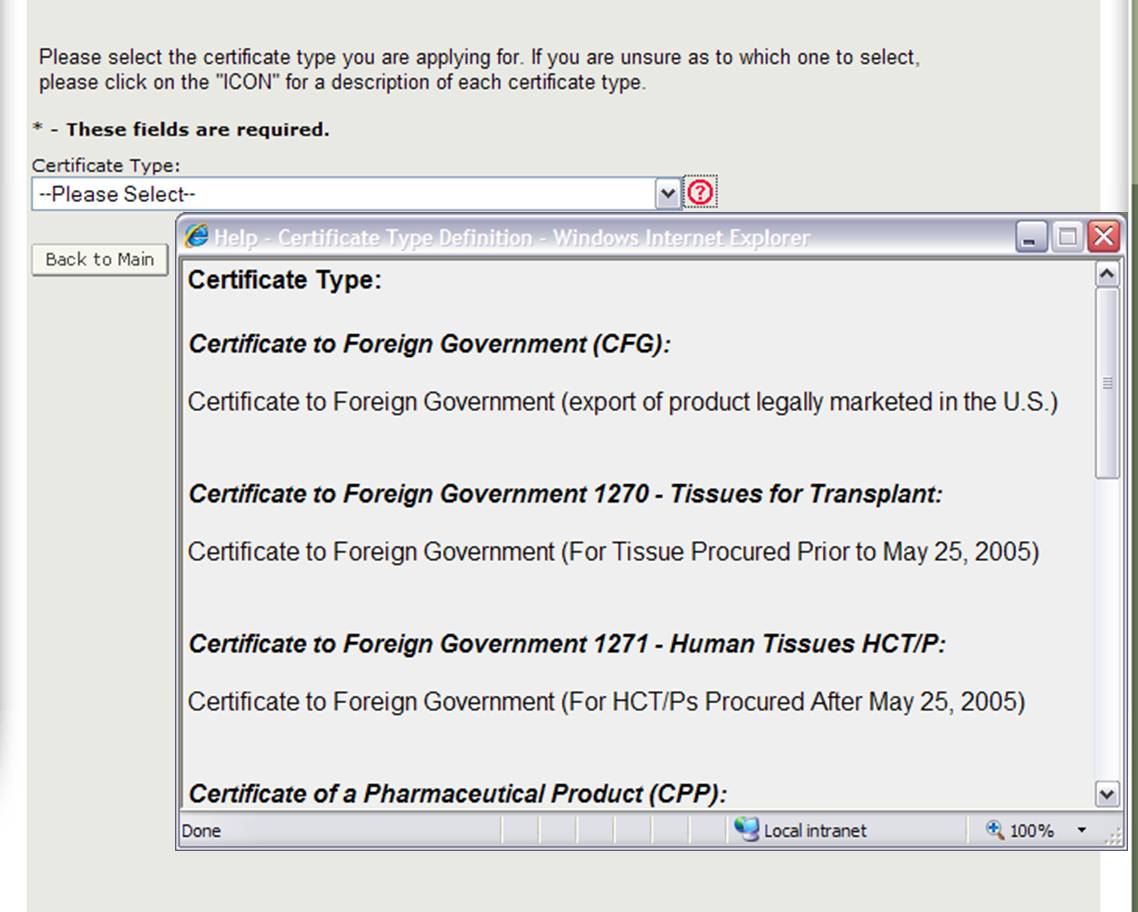
The Center for Biologics Evaluation and Research (CBER) issues several Export Certificate Types. When creating a new application, you will need to first select which certificate type you are requesting as shown in Figure 4.
Figure 4: Certificate Types
NOTE: Currently the Certificate to Foreign Government (CFG) Standard, 1270, and 1271 are the only certificate type available online. The online applications for the other certificate types (which include the Certificate of Pharmaceutical Product, Non-Clinical Research, and Certificate of Exportability) will be available in the near future. Please continue to use the existing paper-based application form for those certificates types.
Select Certificate to Foreign Government (CFG) - Standard
NOTE: For a description of each certificate type, click on the red question mark icon located next to the certificate type list as shown in Figure 4 above.
Description of Certificate Types:
CFG - Standard Certificate to Foreign Government (export of product legally marketed in the U.S.)
CFG - 1270 Certificate to Foreign Government (For Tissue Procured Prior to May 25, 2005)
CFG - 1271 Certificate to Foreign Government (For HCT/Ps Procured After May 25, 2005)
CPP Certificate of a Pharmaceutical Product, World Health Organization (Labeling required)
NCR Non-Clinical Research Use Only Certificate (Export of a non-clinical research use only product, material, or component that is not intended for human use which may be marketed in, and legally exported from the U.S.)
COE (801(e)/802) Certificate of Exportability (For Export of products not approved for marketing in the U.S.)
Simple Notification Simple Notification (Requires persons exporting a drug or device under section 802(b)(1) of the Act to provide a "simple notification identifying the drug or device when the exporter first begins to export such drug or device" to any country listed in section 802(b)(1) of the Act. If the product is to be exported to an unlisted country, section 802(g) of the Act requires the exporter to provide a simple notification "identifying the drug or device and the country to which such drug or device is being exported.)
To view the definitions of the product types for which you can request an Export Certificate in CAP, click on the red question icon located next to the certificate type list. The system will display in a new window with a description of each certificate type as shown in Figure 5 below:
Figure 5: Certificate Type Description
NOTE: At this time the Certificate to Foreign Government (Standard, 1270, and 1271) is the only certificate type that can be requested online. For all other certificate types, please fill out and send the appropriate application form to the following address:
U.S Food and Drug Administration
Center for Biologics Evaluation and Research
Office of Compliance and Biologics Quality
Division of Case Management
10903 New Hampshire Avenue Silver Spring, MD 20993 CBERBECATS@fda.hhs.gov
Navigation
At the top of every page during the Enter New Application process, a status bar will track your progress through each step of the online application process as shown in Figure 6 below.
Figure 6: Navigation Bar
A "Get Help" icon, located at the top right of each step, will provide page specific help. For an overview of all help files available, please refer to the FDA Industry Systems Index of Help Pages at http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/
ComplianceActivities/BiologicsImportingExporting/default.htm
The "FURLS Home" link, located at the top right corner of each page, will take you to the FURLS Home Page. The "BECATS" link, located below the "FURLS Home" link, will take you to the BECATS Main Menu Page (Refer to Figure 1 and Figure 2). To log out of the system, select "FURLS Home" and click on logout.
At the top and bottom of each screen are navigation buttons as shown in Figure 7 below.
Figure 7: General Navigation Buttons
- Back - Go back one screen and continue entering application information. Information entered on the current screen will NOT be saved.
- Save & Exit - Information entered up to this point will be saved. The system will provide you with an application number and your application will be in a "Draft" status in the system for 60 days. After 60 days the application will be deleted from the system. When you log into the CAP system, any applications that are in a "Draft" status will be displayed after selecting the "Enter New Application" option from the main menu.
- Continue - Go to the next screen and continue entering the application form.
- Cancel & Start Again - The system will return you to the Certificate Type selection screen. Refer to Figure 4 above. Any information you have entered will NOT be saved.
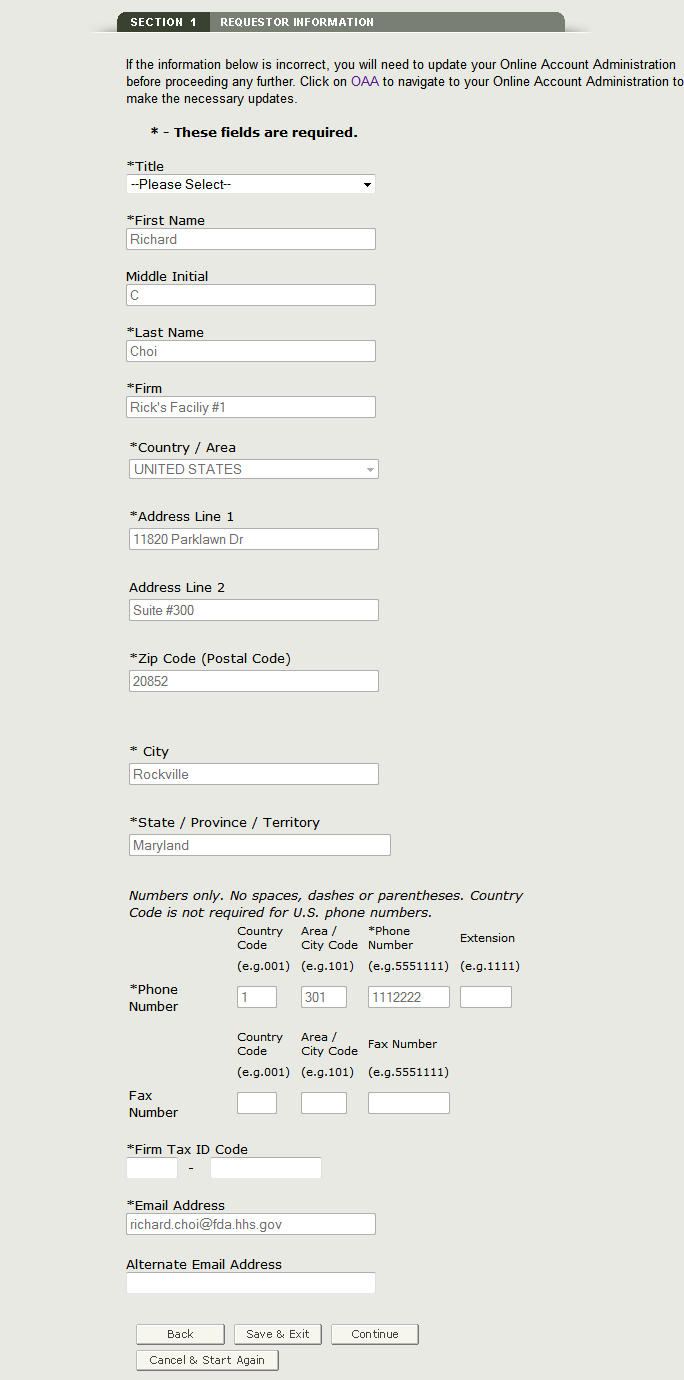
Section 1 Requestor and Billing Information
The requestor is the owner of the account from which the application is filed, and the person requesting the export certificate. The requestor is responsible for completing and signing the application form.
Most of the fields in section one are automatically populated based on the information from your Online Administration Account (OAA) and cannot be edited in BECATS. If the information is incorrect, you can click on the "here" hyperlink and login into your OAA.
You can also click on the "FURLS Home" link, located in the top right corner. Then select "Edit Account Profile" on the left-hand side and update your account profile accordingly. Once you have updated your account, navigate back to BECATS and verify your changes.
Fields marked with an asterisk (*) are mandatory.
NOTE: The following two fields in section 1 are required:
- Title
- Firm Tax ID Code (also referred to as the Employee Identification Number or EIN
(A nine-digit numeric value) - This number is assigned by the Internal Revenue Service (IRS).
You may also enter an optional alternate email address in order to be included on all email notifications for this application.
Once you have completed these fields, click on Continue to Step 2. See Figure 8 below:
Figure 8: Requestor Information
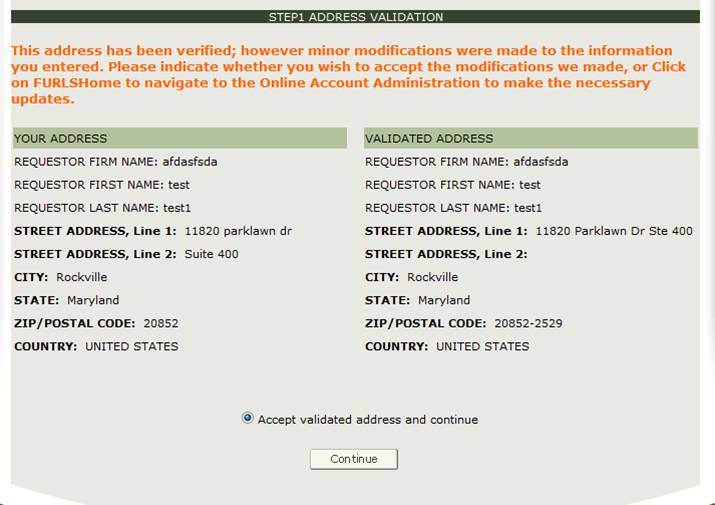
Address Validation
The system perform will perform an address validation. The system will display the "Validated Address" if there are minor differences to the requestor address. If the address is incorrect, you will need to exit the application and make the necessary updates to your Online Account Administration. Otherwise, select the "Accept validated address and continue" radio button and click on Continue to proceed to Step 2. See Figure 9 below.
Figure 9: Address Validation
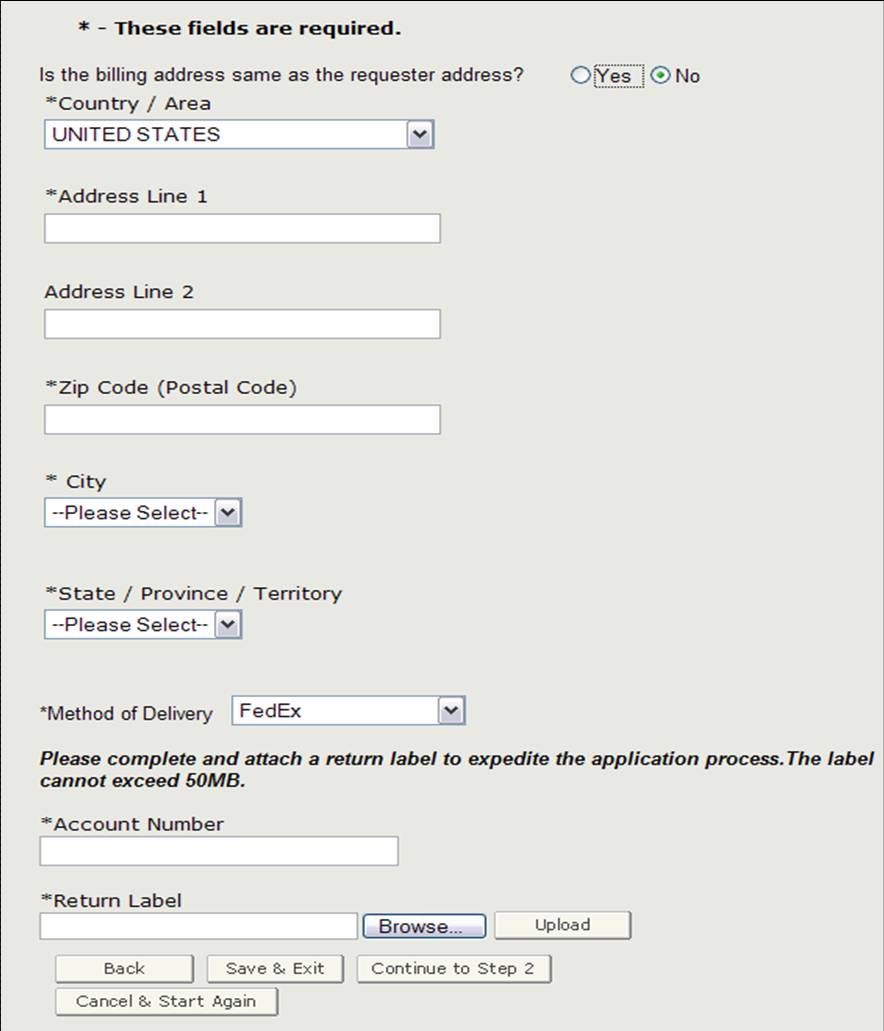
Billing Address / Method of Delivery
Before proceeding to step 2, you will need to verify if the billing address is the same as the requestor address. If it is NOT the same as the requestor address, select "No" and enter the billing address. You will also be able to select the method of delivery. You have the option to select from USPS, FedEx, or UPS. If you do select FedEx or UPS, you will need to provide an account number and attach a filled-out return label as shown in Figure 10.
Figure 10: Billing Address / Method of Delivery
Once you have completed this section, click on "Continue to Step 2".
NOTE: The system will perform an address validation check if you entered a new billing address. The system will display the "Validated Address" if there are minor differences to the billing address. If the address is incorrect, you will need to update the billing address from the previous screen. Otherwise, select the "Accept validated address and continue" radio button and click on Continue to proceed to Step 2.
Section 2
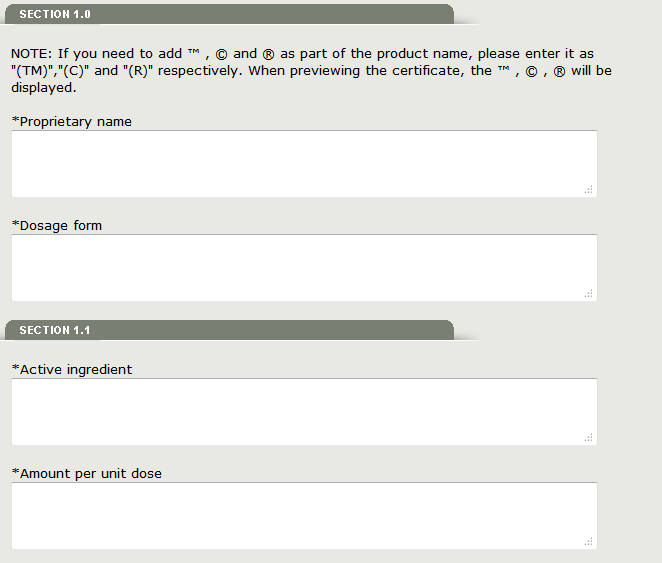
Section 1.0 Proprietary name and Dosage form
Please enter the Proprietary name and Dosage form exactly as you want it to be printed on the certificate.
If you need to add ™, © and ® as part of the product name, please enter it as ‘(TM)’, ‘(C)’ and ‘(R)’ respectively. When previewing the certificate, the ™, ©, ® will be displayed.
Section 1.1 - Active ingredient and Amount per unit dose
Please enter the Active ingredient and Amount per unit dose exactly as you want it to be printed on the certificate.
If you need to add ™, © and ® as part of the product name, please enter it as ‘(TM)’, ‘(C)’ and ‘(R)’ respectively. When previewing the certificate, the ™, ©, ® will be displayed.
NOTE: The Certificate of a Pharmaceutical Product (CPP) is a one page certificate. There is a limit on the number of characters that you can enter for each freeform text field for Sections 1 and 1.1. If you exceed that limit (calculated by the width of each character), you will receive an error message and will need to adjust your entry. If this situation occurs, please abbreviate as much as possible to reduce the number of certificates.
Fill out all fields that have be marked with an asterisk (*) as shown in Figure 11 below.
Figure 11: Section 1 and 1.1 Information
Supporting Document for your Product
The FDA requires at least one supporting document that must be accompanied with this application for the product to be exported. Supporting documents include the Formulation Page, Product Label, or any Product Information. To add a supporting document, click on the ‘Add’ button as shown in Figure 12 below.
Figure 12: Supporting Document
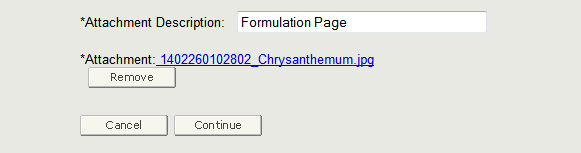
You must enter a description of the attached file in the Attachment Description field. After entering a description, click on the ‘Browse’ button and select the file you wish to upload. Once you have selected the file, click on ‘Upload’. If the system displays the uploaded file in a hyperlink format, then you have successfully attached the file to the application as shown in Figure 13 below.
Figure 13: Browse for a File and Upload
Click on ‘Continue’
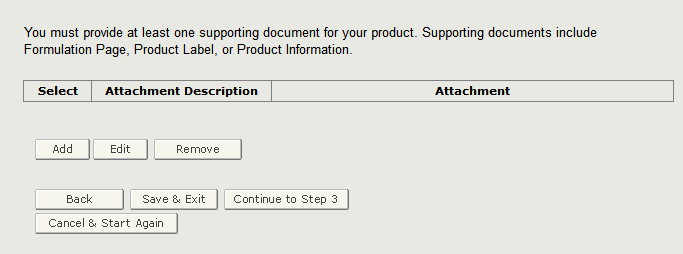
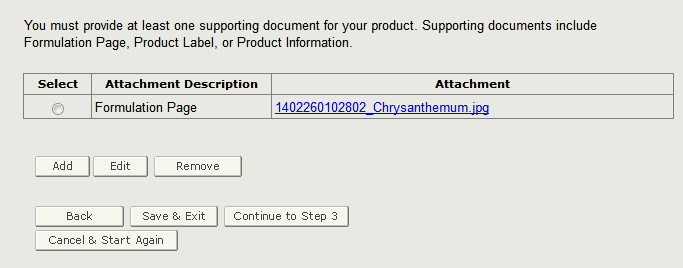
The system displays the Attachment Description along with the uploaded file. If you wish to add additional documents, please click on ‘Add’ and repeat the steps as shown above. You may also remove any existing attached documents by clicking on the radio button next to the Attachment Description and click on ‘Remove’ as shown in Figure 14 below.
Figure 14: Supporting Document Summary Page
Once you have completed this step, proceed to the next step by clicking on ‘Continue to Step 3’.
Section 3
Section 2A.1 & 2A.2 Applicant Address and Marketing Status Number
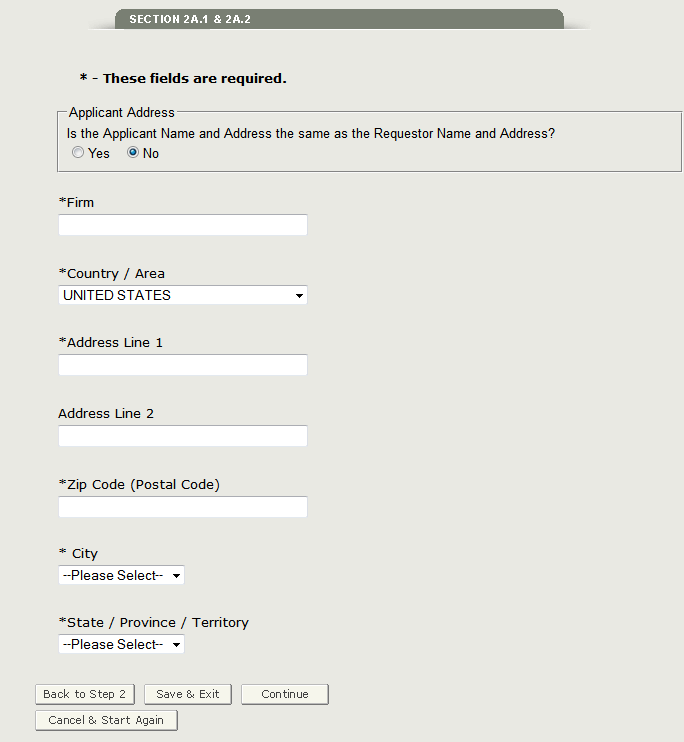
In section 2A.1 you will need to verify if the applicant name and address is the same as the requestor name and address. If it is NOT the same as the requestor name and address, select ‘No’ and enter the applicant name and address as shown in Figure 15 below.
Figure 15: Applicant Address
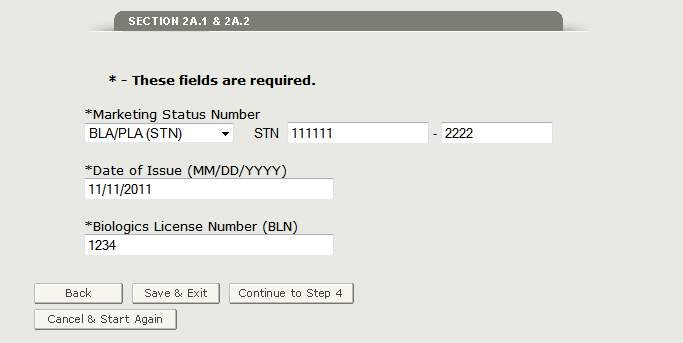
In Section 2A.2, you must enter the type of product in the dropdown menu and the corresponding Marketing Status Number, Date of Issue, and the Biologics License Number (BLN) as shown in Figure 16 below.
Figure 16: Marketing Status Number
Once you have entered all of the fields, click on ‘Continue to Step 4’.
Section 4
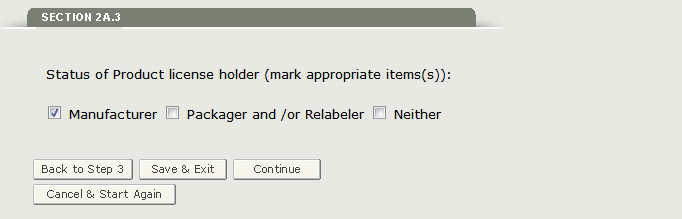
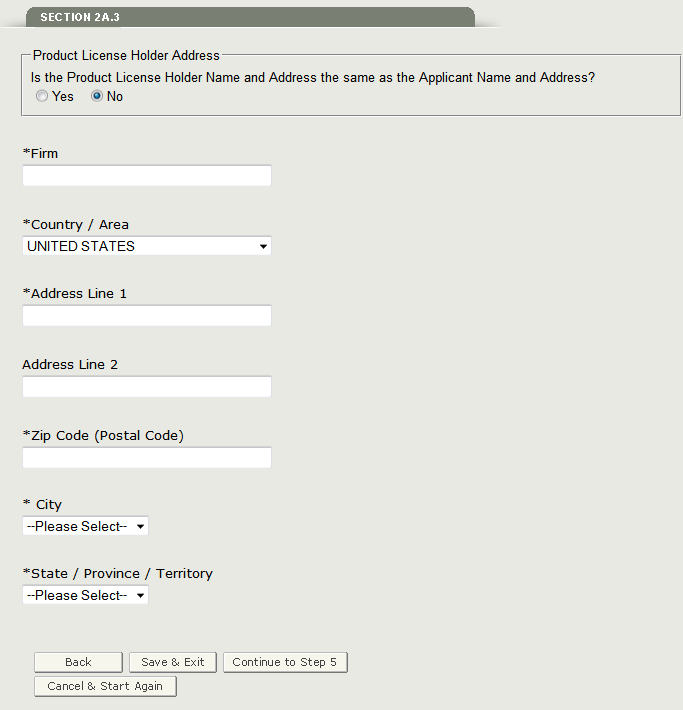
Section 2A.3 - Status of the Product license holder
Please select the status of the product license holder. You have the option to select Manufacturer, Packager and/ or Relabeler, Manufacturer and Packager and/or Relabeler, or Neither as shown in Figure 17 below.
Figure 17: Status of Product license holder
In section 2A.3 you will also need to verify if the product license holder name and address is the same as the applicant name and address. If it is NOT the same as the applicant name and address, select ‘No’ and enter the product license holder name and address as shown in Figure 18 below.
Figure 18: Product license holder name and address
Section 5
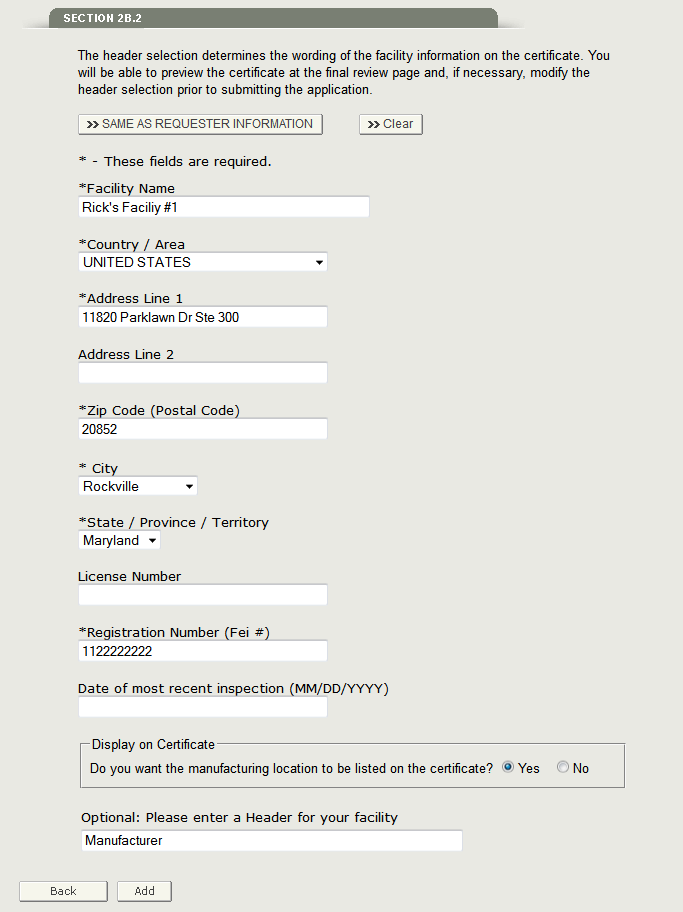
Section 2B.2 Facility Information
In this section, you are required to enter at least one facility on the application. Please enter the facility name, address, and registration number as shown in Figure 19 below.
NOTE: If you select the ‘SAME AS REQUESTOR INFORMATION’ button, the system will populate all required fields except the registration number.
Figure 19: Facility Information
Once you have entered the Firm Name, address, and registration number fields, you must select whether you want the address to be printed on the certificate. If you decide to print the address on the certificate, the system prompts you to optionally enter a header description for the facility to be printed as shown in Figure 19 above.
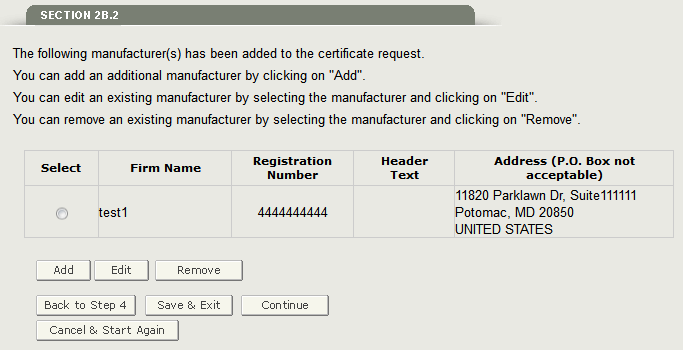
Click on ‘Add’ to add the facility to the application. The system displays the first facility added to your application as shown in Figure 20 below.
Figure 20: Facility List
NOTE: You can add up to five facilities per application.
Add Facility
To add an additional facility, click on the Add button. Enter the required fields and when finished, click on ‘Add’. The system will display the facility added to the facility list.
Edit Facility
To edit a facility, select the radio button next to the facility you wish to edit and click on ‘Edit’ as shown in Figure 20 above. The system will re-display the facility information and allow you to edit any of the fields displayed.
Remove Facility
To remove a facility, select the radio button next to the facility you wish to remove and click on ‘Remove’ as shown in Figure 20 above. The system will display the facility information and a warning message. Click on the ‘Continue’ button to remove the facility from the facility list. You may also select the ‘Cancel’ button if you do not wish to remove the facility.
Once all facilities have been added to the facility list, click on ‘Continue’.
Section 2B.2 Add Remarks
As part of section 2B.2, you have the option to add any remarks you would like to display on the certificate. Please enter any remarks in the freeform text field as shown in Figure 21 below.
Figure 21: Remarks
Click on ‘Continue to Step 6’ to proceed.
Section 6 Importing Countries
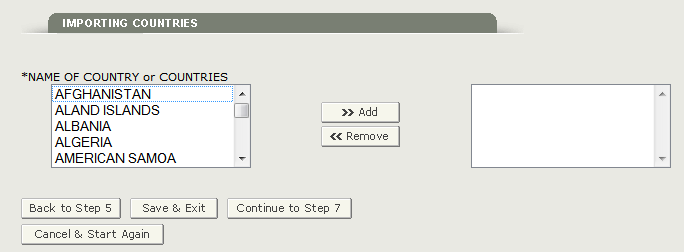
This section is required.
NAME OF COUNTRY or COUNTRIES - Select one or more countries to indicate the product destination as shown in Figure 22 below.
NOTE: Another method to select a country (other than scrolling down the list) is to first click on a country from the country list and then type in the first few letters of the desired country name. The system will jump to the country that begins with the letters typed. You also have the option to hold down the ‘CTRL’ button and select multiple countries.
Figure 22: List of Countries
Once you have made a selection, click on the ‘Continue to Step 7’ button to proceed.
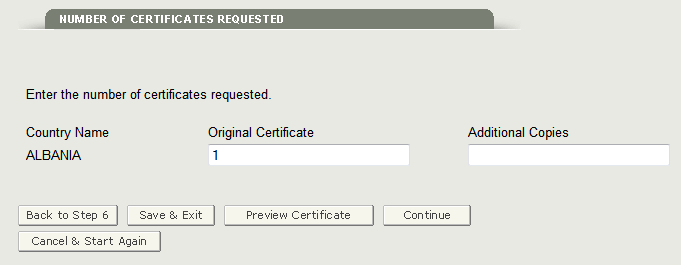
Section 7 Number of Certificates Requested
This section is required.
The system will display the selected country or countries (from step 6) where you will be able to enter additional copies of certificates by country as shown in Figure 23 below.
Figure 23: Number of Certificates Requested by Country
Next, the system displays the total fee that will be billed to you as shown in Figure 24 below.
Figure 24: Total Amount
For more information on how the fee is calculated, click on the ‘How is the fee calculated’ hyperlink.
NOTE: The total number of certificates cannot exceed 50 per application.
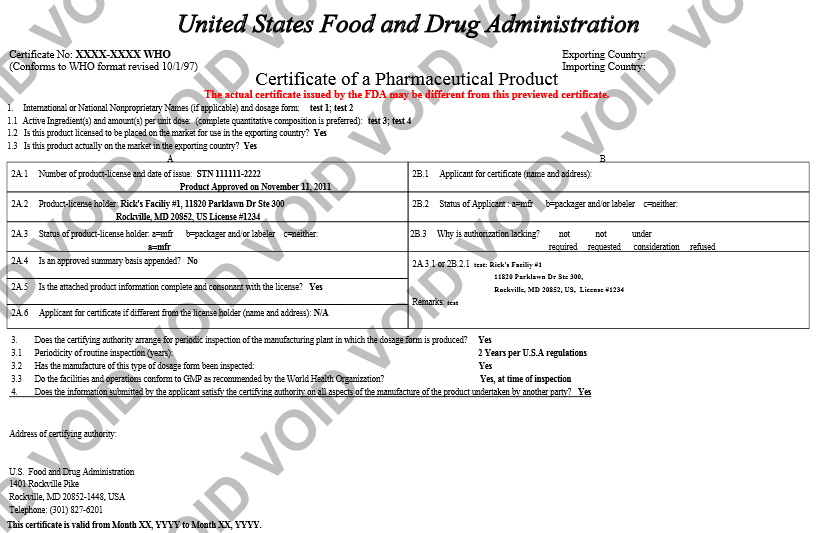
Preview Certificate
Prior to navigating to the next step, the system provides a ‘Preview Certificate’ button. This will allow you to view the certificate (assuming the FDA approves your application). You will be able to view how the certificate will look and, if necessary, make modifications to your application prior to submitting if it is not the expected output.
NOTE: If you find that it is not the desired output, you can modify your application. Specifically, you can perform one or all of the following to your application that will have a direct impact on the display of the certificate:
- Change the manufacturer header information in section 2
- Update the country or countries to be displayed on the certificate in section 6
- Change the number of columns in section 7
- Change the column header information in section 7
- Update the facility or facilities to be displayed on the certificate in section 5
Below is an example of previewing a certificate as shown in Figure 25 below.
Figure 25: Preview Certificate
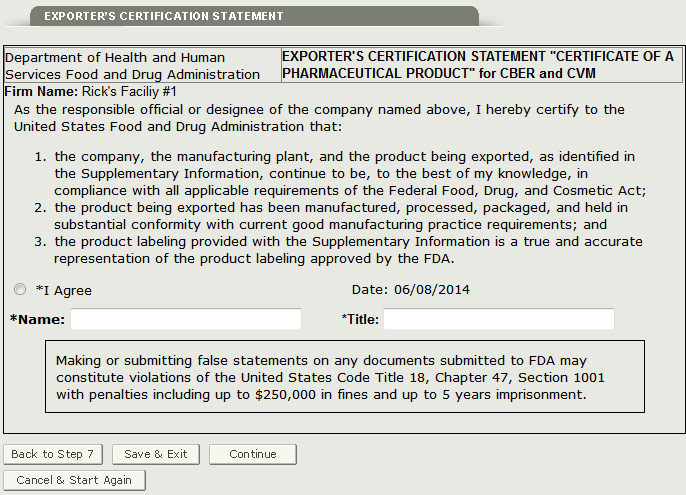
Section 8 Exporter’s Certification Statement (ECS)
The Exporter’s Certification Statement (ECS) acknowledges that you, the responsible official or designee, certify that the facility(s) and the products identified on the Supplemental Information are to the best of your knowledge in substantial compliance with the Federal Food, Drug, and Cosmetic Act (the Act) and all applicable or pertinent regulations.
In this section, the system provides a dropdown list of all facilities entered in section 2 and 3 of the application. You must select one facility from the dropdown list as the primary facility, click on the ‘I Agree’ button located at the bottom of this section, and enter your name and title. You will not be able to continue with the application until these fields have been completed. See Figure 26 below.
Figure 26: Exporter’s Certification Statement
Once you have completed this step, click on the ‘Continue’ button to proceed to the Final Review Page.
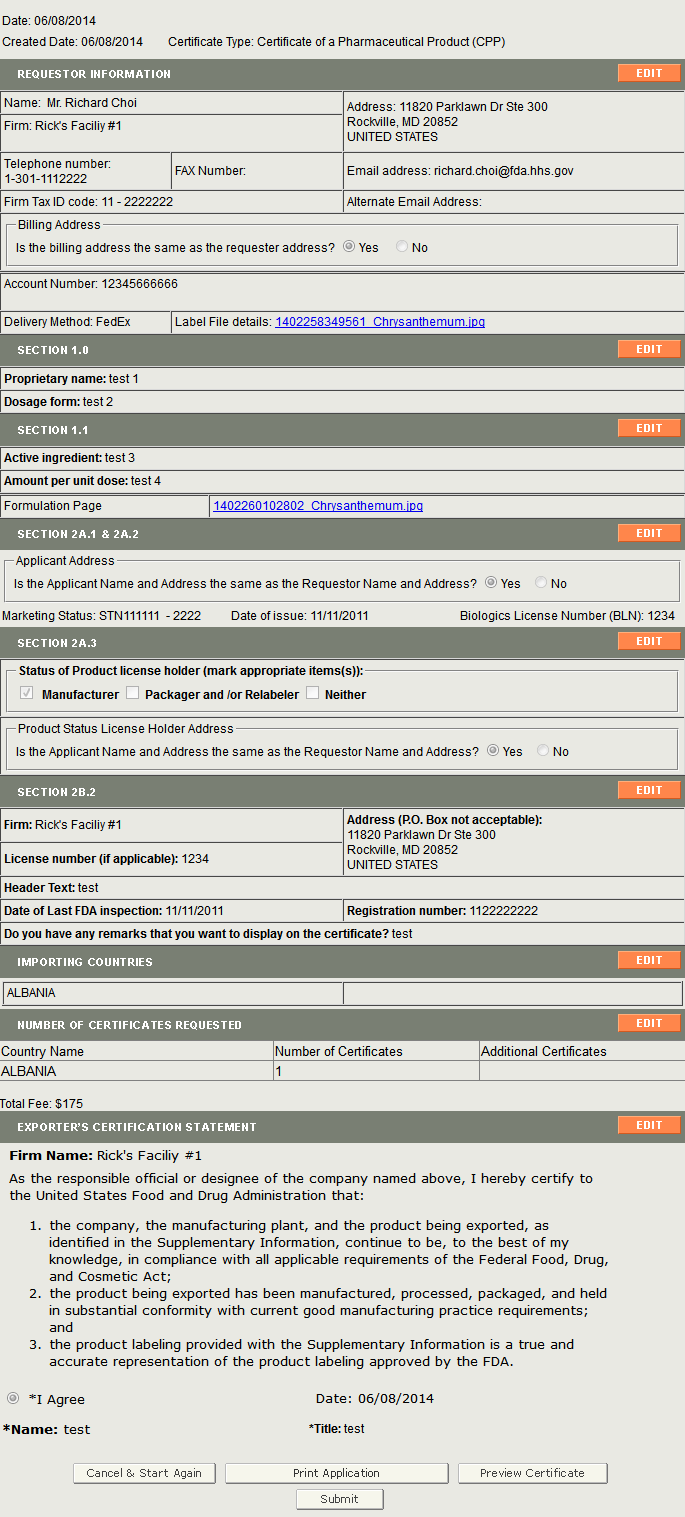
Final Review Screen
The system will display the entire application broken out by section as shown in Figure 27 below. You may choose to modify a section by selecting the ‘Edit’ button next to the step to be updated. The system will re-display the data entry screen corresponding to your chosen section. You may make changes as needed.
Figure 27: Final Review Page
You may choose to print your application prior to submission. Select the ‘Print Application’ button located at the bottom of the review page. A new browser window will open which will allow you to print the application. NOTE: Printing the application will print the contents of the application itself and not a final certification letter. When you are finished, close the browser window in order to return to the BECATS application.
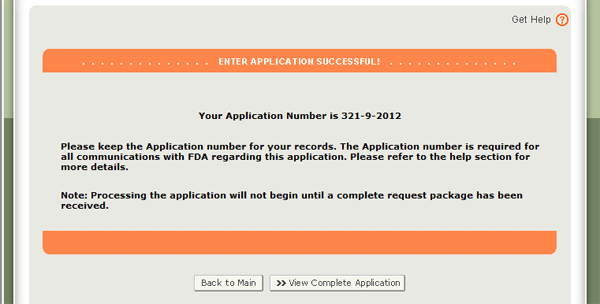
When your application is ready for submission, click on the ‘Submit’ button also located at the bottom of the review page. The system will display a message that your application was successfully submitted as shown in Figure 28 below. The system will provide you with an application number. Please save this number for future reference. The application number will be required to check the status of your application. You will also receive an email confirmation that your application has been successfully received along with the application number.
Figure 28: Submission Page