Drug Trials Snapshots: TEZSPIRE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug. Refer to the TEZSPIRE Prescribing Information for complete information
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
TEZSPIRE (Tezepelumab)
(tez' spyre)
AstraZeneca AB
Approval date: December 17, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TEZSPIRE is used for treatment of severe asthma in adult and pediatric patients aged 12 years and older whose asthma is not well controlled with current medications. TEZSPIRE is to be used in addition to asthma maintenance medications.
How is this drug used?
TEZSPIRE is given once every four weeks by injection under the skin (subcutaneously) by a healthcare provider into the upper arm, thigh, or abdomen.
Who participated in the clinical trials?
The FDA approved TEZSPIRE based on evidence from two clinical trials (NAVIGATOR and PATHWAY) of 1334 patients with severe asthma. The safety and efficacy of TEZSPIRE were evaluated in two clinical trials of patients with severe asthma. The trials were conducted in 24 countries (Argentina, Australia, Austria, Bulgaria, Brazil, Canada, Czech Republic, France, Germany, Hungary, Israel, Japan, Lithuania, Latvia, Russia, Saudi Arabia, Serbia, Slovakia, South Africa, South Korea, Taiwan, Ukraine, United States, and Vietnam).
What are the benefits of this drug?
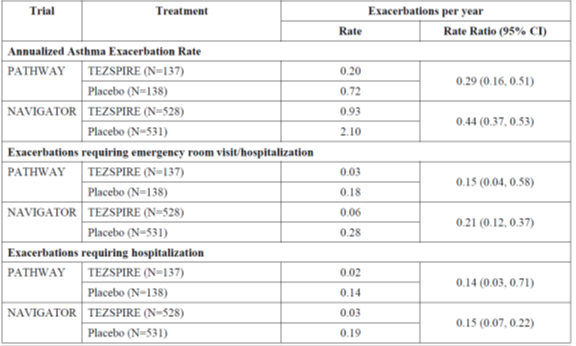
Patients who received TEZSPIRE had fewer asthma attacks that required a stay in the hospital and/or visit to the emergency room.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1. Rate of Asthma Exacerbations during the 52-week Treatment Period in patients with Severe Asthma (NAVIGATOR and PATHWAY trials)a
aRandomized patients
Package Insert
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TEZSPIRE worked similarly in males and females.
- Race: TEZSPIRE worked similarly in White, Asian, and Black or other patients.
- Age: TEZSPIRE worked similarly in all age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2. Asthma Exacerbations Rate Ratio by Age, Sex, and Race (NAVIGATOR and PATHWAY trials)
| Variable | TEZSPIRE N |
Placebo N |
Rate Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| Sex: Female Male |
335 193 |
337 194 |
0.44 0.45 |
0.35, 0.55 0.32, 0.63 |
| Race: White Asian Black or Other |
332 146 50 |
327 149 55 |
0.47 0.36 0.64 |
0.38, 0.58 0.24, 0.56 0.35, 1.20 |
| Age: Adolescents (12 to 17 years) Adults (18-64 years) Adults (>65 years) |
41 391 96 |
41 416 74 |
0.72 0.43 0.42 |
0.39, 1.33 0.35, 0.54 0.26, 0.67 |
FDA Review
What are the possible side effects?
The most common side effects of TEZSPIRE are sore throat, joint aches, and back pain.
What are the possible side effects (results of trials used to assess safety)?
| Adverse Reaction | TEZSPIRE N=665 % |
Placebo N=669 % |
|---|---|---|
| Pharyngitis* | 4 | 3 |
| Arthralgia | 4 | 3 |
| Back pain | 4 | 3 |
The table below summarizes the adverse reactions occurring in patients treated with TEZSPIRE in the NAVIGATOR and PATHWAY trials.
Table 32. Adverse Reactions with TEZSPIRE with Incidence Greater than or Equal to 3% and More Common than Placebo in Patients with Severe Asthma (NAVIGATOR and PATHWAY)
*Pharyngitis (including Pharyngitis, Pharyngitis bacterial, Pharyngitis streptococcal and Viral pharyngitis)
Source: Package Insert
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar among races studied.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 4. Adverse Events (AE) and Serious Adverse Events (SAE) by Sex Subgroup
| Adverse Events | TEZSPIRE (N= 665) | Placebo (N= 669) | ||
|---|---|---|---|---|
| Male (n=243) | Female (n= 422) | Male (n=238) | Female (n=431) | |
| Any AE, n (%) | 180 (74) | 326 (77) | 176 (74) | 354 (82) |
| SAE, n (%) | 26 (11) | 42 (10) | 35 (15) | 58 (14) |
FDA review
Table 5. Adverse Events (AE) and Serious Adverse Events (SAE) by Race Subgroup
| Adverse Events | TEZSPIRE (N= 665) | Placebo (N= 669) | ||||||
|---|---|---|---|---|---|---|---|---|
| White (n=460) | Asian (n = 151) | Black or African American (n = 33) | Other (n= 21) | White (n=450) | Asian (n= 155) | Black or African American (n = 37) | Other (n= 27) | |
| Any AE, n (%) | 345 (75) | 126 (83) | 21 (64 | 14 (67) | 353 (78) | 126 (81) | 31 (84) | 20 (74) |
| SAE, n (%) | 46 (10) | 15 (10) | 6 (18) | 1 (5) | 64 (14) | 20 (13) | 5 (14) | 4 (15) |
FDA review
Table 6. Adverse Events (AE) and Serious Adverse Events (SAE) by Age Subgroup
| Adverse Events | TEZSPIRE (N= 665) | Placebo (N= 669) | ||||
|---|---|---|---|---|---|---|
| 12 to 17 years (n=41) |
18 to 64 years (n=505) |
≥65 years (n=119) |
12 to 17 years (n=41) |
18 to 64 years (n=534) |
≥65 years (n=94) |
|
| Any AE, n (%) | 30 (73) | 387 (77) | 89 (75) | 19 (71) | 425 (80) | 76 (81) |
| SAE, n (%) | 2 (5) | 51 (11) | 15 (13) | 2 (5) | 71 (13) | 20 (21) |
FDA Review
DEMOGRAPHICS SNAPSHOT
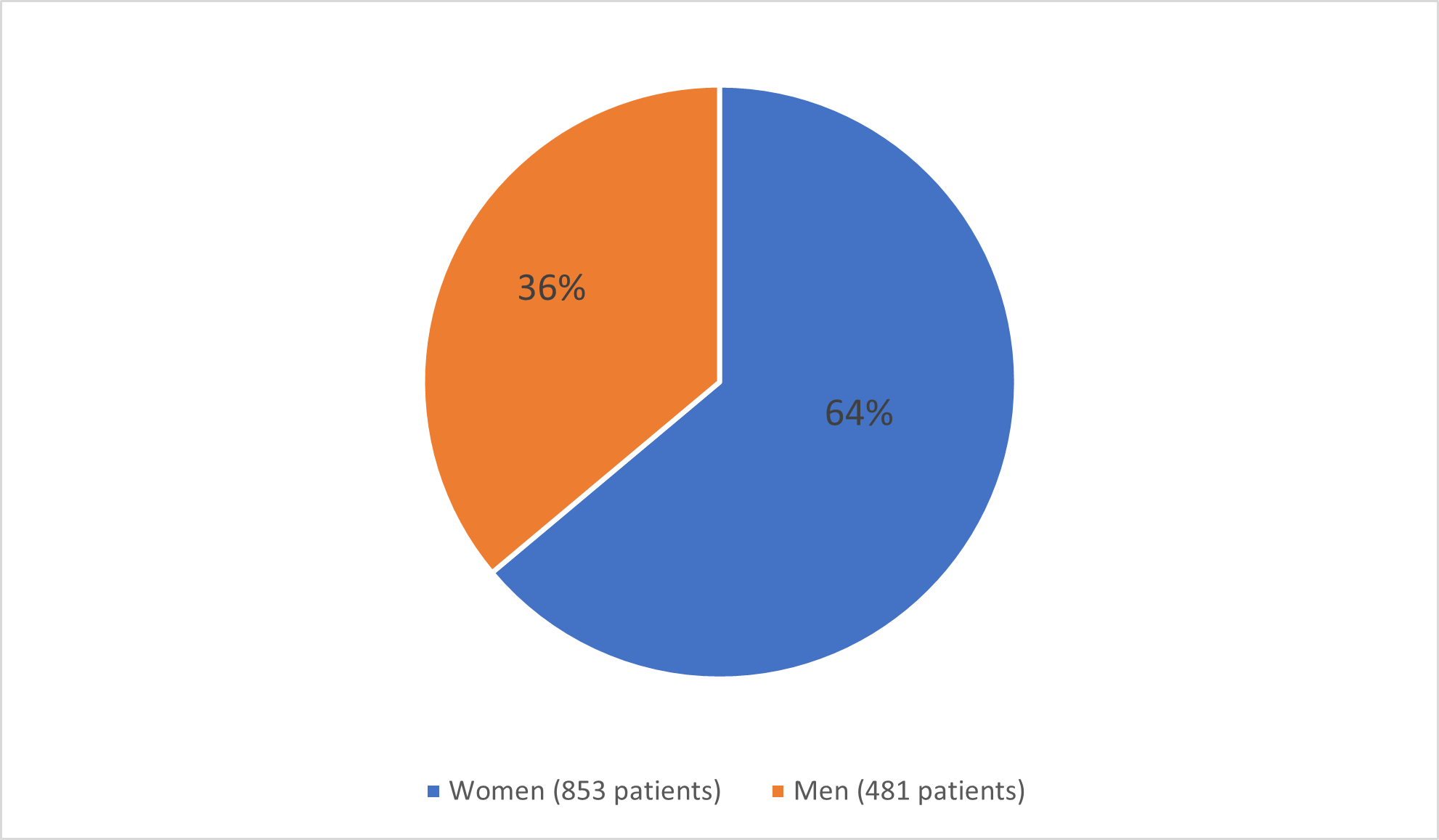
Figure 1 below summarizes how many men and women were enrolled in the clinical trials.
Figure 1. Baseline Demographics by Sex
Adapted from FDA review: Clinical Trial Data
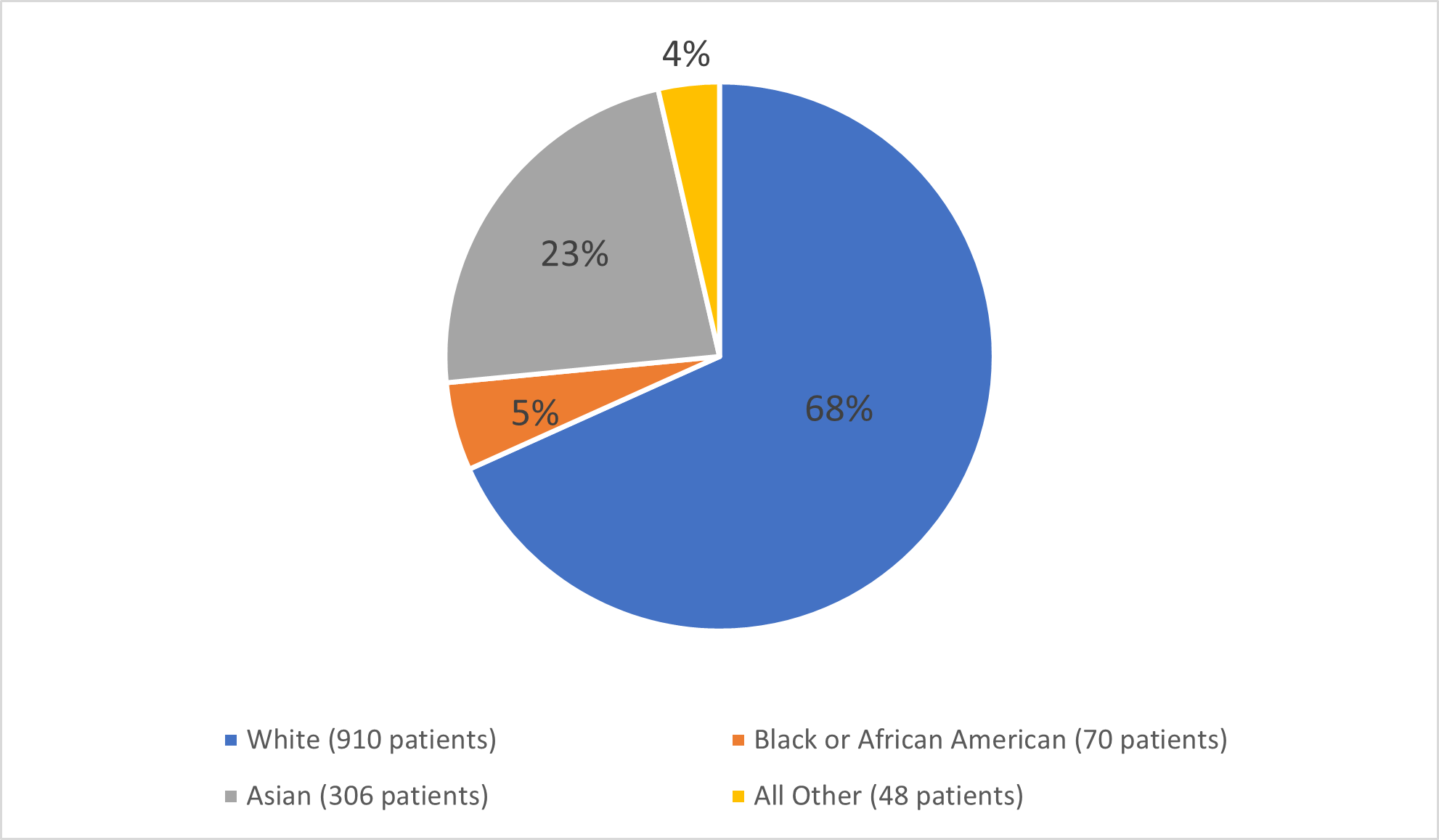
Figure 2 and Table 7 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Adapted from FDA review: Clinical Trial Data
Table 7. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 910 | 68% |
| Asian | 306 | 23% |
| Black or African American | 70 | 5% |
| All Other | 48 | 4% |
Clinical Trial Data
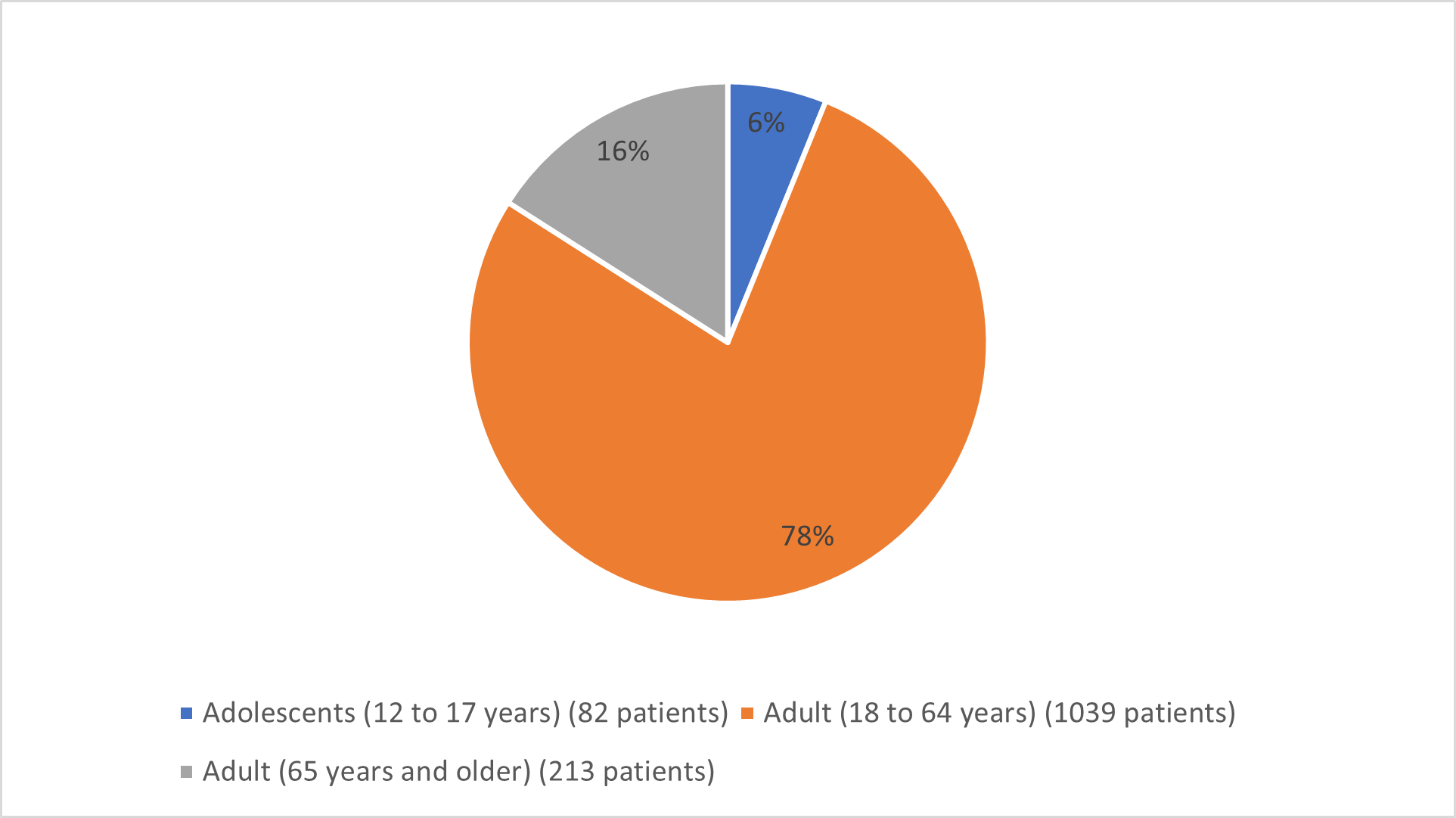
Figure 3 below summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Who participated in the trials?
The FDA approved TEZSPIRE based on evidence from two clinical trials. Combined demographics representing the safety population are presented below.
Table 8. Baseline Demographics of Patients in NAVIGATOR and PATHWAY (Safety Population)
| TEZSPIRE N=665 |
Placebo N=669 |
|
|---|---|---|
| Sex, (n%) | ||
| Female | 422 (64) | 431 (64) |
| Male | 243 (37) | 238 (36) |
| Age, years | ||
| Mean (Standard Deviation) | 51 (16) | 50 (15) |
| Median (min, max) | 53 (12, 80) | 52 (12, 80) |
| Age group, years, (n%) | ||
| Adolescent (12 to 17) | 41 (6) | 41 (6) |
| Adult (18 to 64) | 505 (76) | 534 (80) |
| Adult (≥65) | 119 (18) | 94 (14) |
| Ethnicity, (n%) | ||
| Hispanic or Latino | 84 (13) | 82 (12) |
| Not Hispanic or Latino | 581 (88) | 587 (88) |
| Race, (n%) | ||
| Asian | 151 (23) | 155 (23) |
| Black or African American | 33 (5) | 37 (6) |
| Other | 21 (3) | 27 (4) |
| White | 460 (69) | 450 (67) |
| Country of participation, (n%) | ||
| Argentina | 40 (6) | 41 (6) |
| Australia | 8 (1) | 11 (2) |
| Austria | 2 (<1) | 6 (1) |
| Bulgaria | 14 (2) | 18 (3) |
| Brazil | 47 (7) | 46 (7) |
| Canada | 19 (3) | 17 (3) |
| Czech Republic | 16 (2) | 9 (1) |
| Germany | 56 (8) | 47 (7) |
| France | 20 (3) | 21 (3) |
| Hungary | 27 (4) | 22 (3) |
| Israel | 33 (5) | 28 (4) |
| Japan | 63 (10) | 45 (7) |
| Korea | 54 (8) | 72 (11) |
| Lithuania | 0 (0) | 3 (<1) |
| Latvia | 7 (1) | 9 (1) |
| Russia | 25 (4) | 26 (4) |
| Saudi Arabia | 5 (1) | 2 (<1) |
| Serbia | 2 (<1) | 2 (<1) |
| Slovakia | 14 (2) | 19 (3) |
| Taiwan | 5 (1) | 4 (1) |
| Ukraine | 47 (7) | 45 (7) |
| United States | 102 (15) | 103 (15) |
| Vietnam | 8 (1) | 12 (2) |
| South Africa | 51 (8) | 61 (9) |
Clinical Trial Data
How were the trials designed?
The benefits and side effects of TEZSPIRE were evaluated in two clinical trials of patients with severe asthma. All patients were taking their usual treatment for asthma. In addition, patients received new treatment with either TEZSPIRE or placebo. Neither the patients nor the investigators knew which treatment was given. The benefit of TEZSPIRE was assessed by measuring the frequency of asthma attacks (exacerbations) at the end of both 52 week trials in comparison to placebo.
How were the trials designed?
The efficacy and safety of TEZSPIRE were evaluated in two randomized, double-blind, parallel-group, placebo-controlled trials (NAVIGATOR and PATHWAY) in patients with severe asthma whose asthma was not well controlled with their usual asthma medications. NAVIGATOR enrolled adult and pediatric patients 12 years of age and older, while PATHWAY enrolled adults. Patients continued with their usual asthma therapy throughout the duration of the trials. Patients were enrolled without requiring a minimum baseline level of blood eosinophils or fractional exhaled nitric oxide (FeNO).
Patients received either TEZSPIRE or a matched placebo control every 4 weeks for 52 weeks.
The efficacy outcome measure in NAVIGATOR and PATHWAY was the frequency of asthma exacerbations for each patient during the 52-week trials. Asthma exacerbation was defined as a worsening of asthma requiring use of oral/systemic corticosteroids for at least 3 days, and/or emergency department visits requiring use of oral/systemic corticosteroids and/or hospitalization.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.