Division of Applied Regulatory Science | Sampling of DARS Projects
Over-the-Counter Drug Research
Evaluation of Sunscreen Active Ingredients
DARS has conducted two randomized clinical trials which demonstrated systemic exposure of multiple commonly used sunscreen active ingredients under single and maximal use conditions. Additionally, DARS is evaluating in vitro metabolism and transporter-based drug interactions with sunscreen active ingredients. The clinical effect of these findings is unknown and continued use of sunscreen is encouraged.
Publications
Abbasi J. FDA Trials Find Sunscreen Ingredients in Blood, but Risk Is Uncertain. JAMA 2020, 323(15):1431–1432.
FDA Spotlight on CDER Science: New FDA Study Shines Light on Sunscreen Absorption. July 2019.
FDA Voices: Shedding More Light on Sunscreen Absorption. January 2020.
Matta et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2019, 321(21):2082-91.
Matta et al. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2020, 323(3):256-67.
Pre-Market Research
Comprehensive in vitro Proarrhythmia Assay (CiPA)
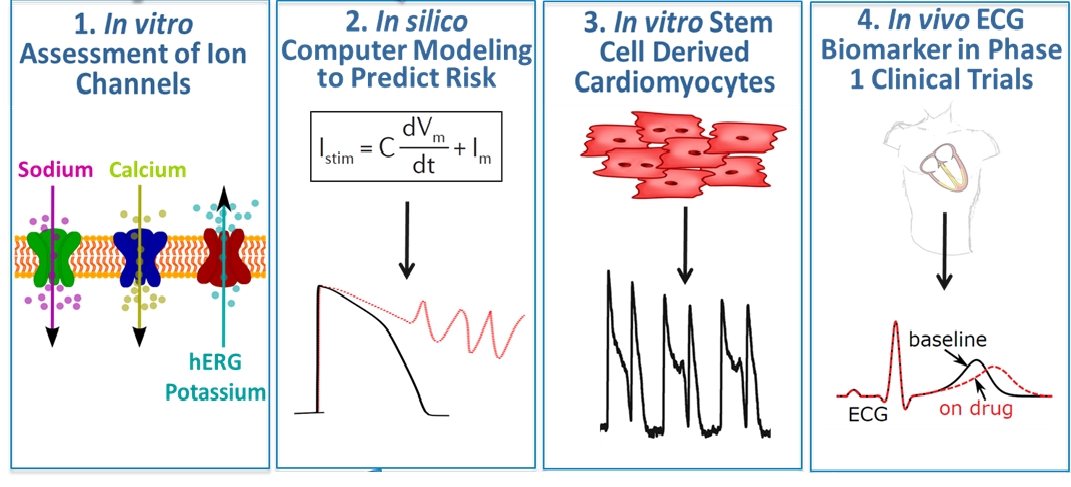
DARS is working with an international consortium on the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, which is developing an in vitro (cellular models) and in silico (computational) paradigm for cardiac safety evaluation of new drugs. CiPA involves four components: (1) in vitro assessment of drug effects on multiple cardiac ion channels; (2) integration of the multi-ion channel effects in an in silico computer model of the human ventricular cardiomyocyte (heart cells) to output a proarrhythmic (abnormal heart rhythm) risk score; (3) assessment for unanticipated effects in vitro using human-induced pluripotent stem cell-derived cardiomyocytes; and (4) phase 1 clinical studies with exposure-response analysis. DARS is leading applied research across all four components to develop and validate this novel regulatory paradigm.
Integrated Cellular Systems
DARS is currently evaluating liver, heart, and connected liver and heart microphysiological systems to determine if they are suitable for drug development, including prediction of adverse events and metabolism. Additionally, both hepatocytes and cardiomyocytes are currently being engineered from stem cells. The cardiomyocytes are microengineered at the single-cell and tissue levels so they are more similar to mature cardiomyocytes compared to more traditional methods. The hepatocytes are being evaluated in both planar and spheroid form to determine how their function and reaction to toxicity compares to primary human hepatocytes.
Publications
Blinova et al. Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs. Clin Trans Sci 2019, 12(6):687-97.
FDA Impact Story: Improved Assessment of Cardiotoxic Risk in Drug Candidates: The Comprehensive in vitro Proarrhythmia Assay. March 2020.
Indapurkar et al. Simultaneous UHPLC-MS/MS method of estradiol metabolites to support the evaluation of Phase-2 metabolic activity of induced pluripotent stem cell derived hepatocytes. J Chromatogr B Analyt Technol Biomed Life Sci 2019, 1126-27:121765.
Li et al. Quantitative Systems Pharmacology Models for a New International Cardiac Safety Regulatory Paradigm: An Overview of the Comprehensive In Vitro Proarrhythmia Assay In Silico Modeling Approach. CPT Pharmacometrics Syst Pharmacol 2019, 8(6):371-9.
Li et al. General Principles for the Validation of the Proarrhythmia Risk Prediction Models: An Extension of the CiPA In Silico Strategy. Clin Pharmacol Ther 2020, 107(1):102-11.
Matta et al. Novel High-Throughput Quantitation of Potent hERG Blocker Dofetilide in Human Plasma by Liquid Chromatography Tandem Mass Spectrometry: Application in a Phase 1 ECG Biomarker Validation Study. J Anal Toxicol 2019, Epub.
Ribeiro et al. Liver Microphysiological Systems for Predicting and Evaluating Drug Effects. Clin Pharmacol Ther 2019, 106(1):139-47.
Ribeiro et al. Considerations for an In Vitro, Cell-Based Testing Platform for Detection of Drug-Induced Inotropic Effects in Early Drug Development. Part 2: Designing and Fabricating Microsystems for Assaying Cardiac Contractility With Physiological Relevance Using Human iPSC-Cardiomyocytes. Front Pharmacol 2019, 10:934.
Wu et al. Drug potency on inhibiting late Na+ current is sensitive to gating modifier and current region where drug effects were measured. J Pharmacol Toxicol Methods 2019, 100:106605.
Biosimilar and Generic Drug Research
Pharmacodynamic Biomarkers for Biosimilar Approval
To ensure US patients realize the public health benefit of a robust, competitive market for biosimilar products, FDA is focused on improving the efficiency of biosimilar development. DARS is conducting research to inform FDA’s thinking on critical aspects of the use of pharmacodynamic biomarkers to demonstrate biosimilarity, which can either streamline or negate the need for comparative clinical studies. This project will result in an evidentiary framework and methodology for identifying, characterizing and applying biomarkers to serve as primary clinical assessment for biosimilar approval. Three clinical studies are being performed to characterize biomarkers for biosimilar development.
Biologics/Biosimilars and Complex Generics Safety, Quality, and Availability Studies
DARS is utilizing a wide range of studies, including in vitro and in vivo methodologies, to evaluate the safety, quality, and availability of biologics, biosimilars, and generics. Humanized mouse models are being utilized to evaluate immune-mediated response to biologic products and peptide generic drugs. Additionally, in vitro methods are being used to evaluate the suitability of multiple bioassays for PEGylated products to better address the challenges associated with determining biosimilarity. Finally, an in vivo model to evaluate a dexamethasone intravitreal implant will determine if systemic dexamethasone exposure may serve as a surrogate biomarker to assess bioequivalence of generic implant products.
Publications
Li et al. Advancing Biosimilar Development Using Pharmacodynamic Biomarkers in Clinical Pharmacology Studies. Clin Pharmacol Ther 2020; 107:40-42.
Semple et al. Evaluation of the Ability of Immune Humanized Mice to Demonstrate CD20-Specific Cytotoxicity Induced by Ofatumumab. Clin Transl Sci 2019, 12(3):283-90.
Weaver et al. BLT-Immune Humanized Mice as a Model for Nicolumab-Induced Immune-Mediated Adverse Events: Comparison of the NOG and NOG-EXL Strains. Toxicol Sci 2019, 169(1):194-208.
Yan et al. Bone marrow-liver-thymus (BLT) immune humanized mice as a model to predict cytokine release syndrome. Transl Res 2019, 210:43-56.
Yan et al. Evaluation of a TGN1412 analogue using in vitro assays and two immune humanized mouse models. Toxicol Appl Pharmacol 2019, 372:57-69.
Drugs with Abuse Potential Research
Clinical Study on the Combination of Psychotropic Drugs and Opioids on Respiratory Depression
FDA recently added black box warning to drug labels for opioids and benzodiazepines to not co-administer due to risk for respiratory depression; it is unclear whether other psychotropic drugs can have a similar effect when co-administered with opioids. The primary objective of this project is to study whether two psychotropic drugs increase opioid-induced respiratory depression when co-administered.
Computational Models and in vitro Studies
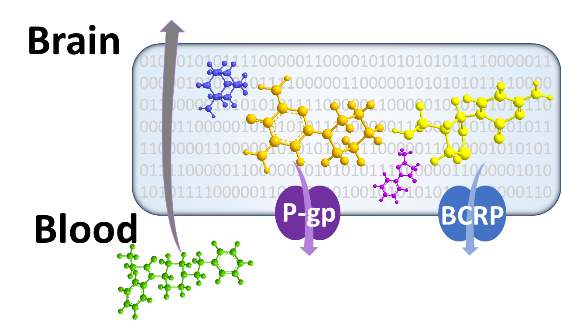
DARS uses multiple computational and in vitro methodologies to predict the risk to public safety of newly-identified compounds. For example, a quantitative structure-activity relationship model was recently created to predict if a chemical will cross the blood-brain barrier, therefore potentially leading to neurotoxic effects. Additionally, a model is currently under development to predict the dose of naloxone needed to overcome opioid-induced respiratory depression. Finally, an in vitro study is evaluating if opioids can block cardiac ion channels and how that affects QTc prolongation.
Publications
FDA Impact Story: Preclinical Research to Achieve Safer Prescribing of Psychoactive Therapeutics for Patients Who Use Opioids. February 2019.
Xu et al. Developing an animal model to detect drug-drug interactions impacting drug-induced respiratory depression. Toxicol Rep 2020, 7:188-97.
Post-Market Research
Assessing Drug Combination Strategies to Reduce Antimicrobial Resistance
DARS has developed an in vitro method to quantitate the rate at which antibiotic resistance appears. The system uses a hollow fiber bioreactor system to deliver single drugs or combinations of drugs using human pharmacokinetic profiles. Additionally, next-generation sequencing is used to investigate the genetic and epigenetic biomarkers of antibiotic resistance, and genomics and bioinformatics are utilized to investigate the effects of oral antibiotics on in vitro bacterial genomes and in vivo gut microbiome.
Publications
Chockalingam et al. Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model for the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection. Antibiotics (Basel) 2019, 8(4):E170.
Gandhi et al. Combining LC-MS/MS and hollow-fiber infection model for real-time quantitation of ampicillin to antimicrobial resistance. Future Sci OA 2018. 5(1):FSO349.
(Quantitative) Structure Activity Relationship Consult Service
DARS chemical informatics projects (using computational techniques to solve chemistry problems) are focused on endpoints of regulatory interest, linked through chemical structure. Research activities include (1) database development for model training and validation; (2) development of web-based applications to enable direct structure-based searching by CDER staff; and (3) (Q)SAR model development (models using physical and chemical properties to predict the behavior of new drugs) to support drug safety review. Databases and models are used to respond to regulatory review consultation requests.
Publications
Amberg, et al. Extending (Q)SARs to incorporate proprietary knowledge for regulatory purposes: is aromatic N-oxide a structural alert for predicting DNA-reactive mutagenicity? Mutagenesis 2019, 34(1):67-82.
FDA Impact Story: CDER Assessment of Drug Impurity Mutagenicity by Quantitative Structure-Activity Relationship Modeling. May 2020.
Hasselgren, et al. Genetic toxicology in silico protocol. Regul Toxicol Pharmacol 2019, 107:104403.
Landry et al. Transitioning to composite bacterial mutagenicity models in ICH M7 (Q)SAR analyses. Regul Toxicol Pharmacol 2019, 109:104488.