Patient-Focused Drug Development
Twitter: #OCEPFDD
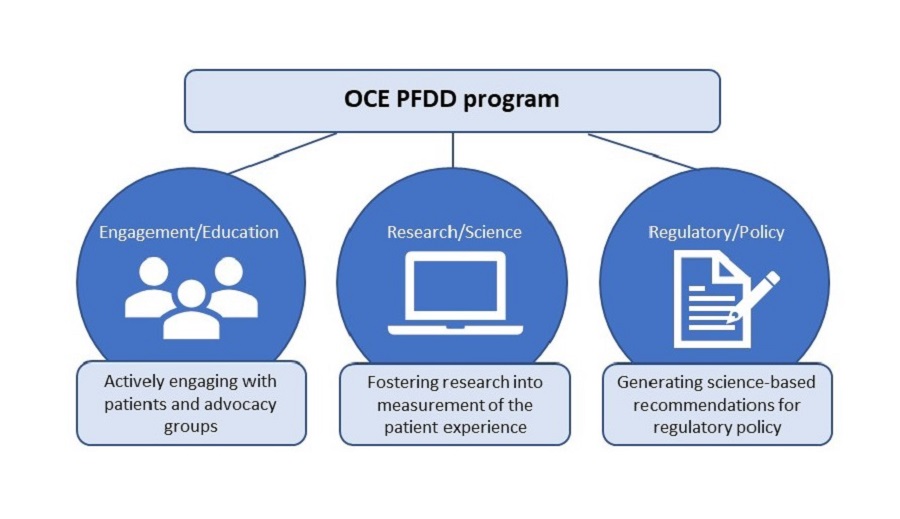
The Oncology Center of Excellence Patient-Focused Drug Development (PFDD) program fosters collaboration between FDA Centers and external stakeholders involved in patient outcomes research in cancer populations. The program focuses on three key areas:
- Engagement/Education
- Research/Science
- Regulatory/Policy
Cancer patients experience disease symptoms and symptomatic treatment side effects that can impact their ability to function and other aspects of their health-related quality of life. The PFDD Program is working to identify rigorous methods to assess the patient experience that will complement existing survival and tumor information to provide additional evidence about the effects of cancer therapies on patients.
Paul G. Kluetz, M.D.
Oncology Center of Excellence
Paul Kluetz is a medical oncologist and the Deputy Director of the Oncology Center of Excellence at the U.S. FDA. In addition to his broader role in OCE’s strategic oversight and management, Dr. Kluetz founded the OCE’s PFDD program and continues to support its strategic mission. He has engaged the global cancer drug development and health outcomes community in leading a sustained effort to advance patient reported outcomes (PRO) data, wearable technologies, and other methods to obtain rigorous patient experience data in both the clinical trial and “real-world” settings.
Vishal Bhatnagar, M.D.
Oncology Center of Excellence
Vishal Bhatnagar, MD, is a medical oncologist/hematologist and the Associate Director for Patient Outcomes in the OCE. His interests include patient reported outcomes, patient preference and incorporation of patient experience in oncology trials. His work focuses on the operational management of the OCE’s Patient-Focused Drug Development program. Additionally, Dr. Bhatnagar has a strong clinical interest in multiple myeloma and has previously served as an Office of Hematology and Oncology multiple myeloma scientific liaison. Dr. Bhatnagar received his BA in Political Science and his medical degree at the George Washington University. He completed his internal medicine residency and hematology/oncology fellowship at the University of Maryland.
Erica Horodniceanu, MPH
Oncology Center of Excellence
Erica Horodniceanu, MPH, is a health scientist in the OCE’s Patient-Focused Drug Development program at the FDA. Over the past 18 years, Erica has provided healthcare research, health education, health communications, and project management services to pharmaceutical, biotech, and medical device companies. She has previously held positions in industry within consulting firms focused on outcomes research and has been working in the field of clinical outcome assessments (COAs) for the past 8 years. Erica holds a Bachelor of Science degree in Health Science Education, with a concentration in Health Promotion from the University of Florida and a Master’s in Public Health degree in Public Health Practice and Policy from the University of Maryland.
Education/Engagement:
- Create educational opportunities for the patient community
- Engage with patient advocacy groups in patient-centered research
- Foster educational opportunities for FDA oncology reviewers related to measurement of symptoms and function
Research/Science:
- Engage and participate with international scientific research in PRO measurement with international organizations
- Identify analyses and visualizations to interpret clinical outcome data from cancer trials
- Actively engage in scientific collaboration with clinicians, social scientists, and statisticians across FDA and externally with the academic community
Regulatory/Policy:
- Create standard analyses for physical function and symptomatic adverse events
- Foster efficient regulatory review of clinical outcome assessments in oncology regulatory submissions
- Develop guidance for industry
Projects
- Collaborator: Kaiser Permanente, Division of Research – PANcreatic cancer Patient Reported Outcomes using the Electronic medical record (PanPROE)
- Collaborator: Northwestern University and University of Tasmania – Evaluation of a global item for side effect bother
- Collaborator: Duke University - Evaluating physical functioning using patient-reported outcome measures: How does the question form and recall period influence patients’ interpretation?
- Collaborator: Mayo Clinic and Yale University - Quantifying physical function in cancer patients undergoing chemotherapy using clinician- and patient-reports along with wearable device data
- Collaborator: LUNGevity Foundation - Understanding the lung cancer Patient ExperiEnce in the Real-world setting and comparing it to the regulatory setting (Project PEER)
- Collaborator: National Cancer Institute– Advancing the use of digital health technologies and digital symptom reporting for patient-reported outcome measures
Working Groups
- National Cancer Institute (NCI) Cancer Moonshot Tolerability Consortium Steering Committee
- Patient-Reported Outcomes Tools: Engaging Users & Stakeholders (PROTEUS) consortium
- Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL)
- Standard Protocol Items: Recommendations for Interventional Trials Patient-Reported Outcomes (SPIRIT-PRO)
Upcoming Events:
June 29, 2022: 7th Annual Clinical Outcomes Assessment in Clinical Cancer Trials (COA-CCT) Workshop
Past Events:
Clinical Outcomes Assessment in Clinical Cancer Trials (COA-CCT) Workshops
July 21 and 23, 2021: 6th COA-CCT Workshop
July 17, 2020: 5th COA-CCT Workshop with American Society of Clinical Oncology
July 12, 2019: 4th COA-CCT Workshop with American Society of Clinical Oncology
June 22, 2018: 3rd COA-CCT Workshop with American Society for Clinical Oncology
April 25, 2017: 2nd COA-CCT Workshop with the Critical Path Institute
April 26, 2016: COA-CCT Workshop with the Critical Path Institute
Other Events
May 11-12, 2021: Meeting of the Pediatric Oncology Subcommittee of the Oncologic Drugs Advisory Committee
October 8, 2019: 3rd Partners in Progress
November 27, 2018: 2nd Partners in Progress
November 13, 2017: 1st Partners in Progress
The OCE PFDD program has published multiple manuscripts in peer-reviewed journals to advance patient-focused drug development. Some selected articles are listed below:
FDA review summary of patient-reported outcome results for ibrutinib in the treatment of chronic graft versus host disease.
King-Kallimanis, B.L., Wroblewski, T., Kwitkowski, V., De Claro, R.A., Gwise, T., Bhatnagar, V., et al.
Qual. Life Res. 2020. 10.1007/s11136-020-02448-y
Patient‐Reported Outcomes in Oncology Clinical Trials: Stakeholder Perspectives from the Accelerating Anticancer Agent Development and Validation Workshop 2019.
Bhatnagar, V., Hudgens, S., Piault-Louis, E., Jones, L., Beaver, J., Lyerly H.K., Reaman, G., Fleming, T., Kluetz, P.
The Oncologist. 2020. doi.org/10.1634/theoncologist.2020-0062
Patient-Reported Outcomes in Anti-PD-1/PD-L1 Inhibitor Immunotherapy Registration Trials: FDA Analysis of Data Submitted and Future Directions.
King-Kallimanis BL, Howie LJ, Roydhouse JK, Singh H, Theoret MR, Blumenthal GM, Kluetz PG.
Clinical Trials. 2019. doi.org/10.1177/1740774519836991
FDA Approval Summary: Pertuzumab for adjuvant treatment of HER2-positive early breast cancer.
Howie LJ, Scher NS, Amiri-Kordestani L, Zhang L, King-Kallimanis BL, Choudhry Y, Schroeder J, Goldberg KB, Kluetz PG, Ibrahim A, Sridhara R, Blumenthal GM, Pazdur R, Beaver JA.
Clin Cancer Res. 2018 Dec 14. pii: clincanres.3003.2018. doi: 10.1158/1078-0432.CCR-18-3003. [Epub ahead of print]
Blinding and Patient-Reported Outcome Completion Rates in US Food and Drug Administration Cancer Trial Submissions, 2007-2017.
Roydhouse JK, King-Kallimanis BL, Howie LJ, Singh H, Kluetz PG.
J Natl Cancer Inst. 2018 Dec 17. doi: 10.1093/jnci/djy181. [Epub ahead of print]
Learning from Patients: Reflections on Use of Patient-Reported Outcomes in Lung Cancer Trials.
Basu Roy U, King-Kallimanis BL, Kluetz PG, Selig W, Ferris A.
J Thorac Oncol. 2018 Dec;13(12):1815-1817. doi: 10.1016/j.jtho.2018.09.003. Epub 2018 Oct 15. No abstract available.
Moving forward toward standardizing analysis of quality of life data in randomized cancer clinical trials.
Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Dueck AC, Devlin N, Flechtner HH, Gotay C, Greimel E, Griebsch I, Groenvold M, Hamel JF, King M, Kluetz PG, Koller M, Malone DC, Martinelli F, Mitchell SA, Moinpour CM, Musoro JZ, O'Connor D, Oliver K, Piault-Louis E, Piccart M, Pimentel FL, Quinten C, Reijneveld JC, Schürmann C, Smith AW, Soltys KM, Sridhara R, Taphoorn MJB, Velikova G, Coens C.
Clin Trials. 2018 Dec;15(6):624-630. doi: 10.1177/1740774518795637. Epub 2018 Aug 24.
Overview of Oncology and Hematology Drug Approvals at US Food and Drug Administration Between 2008 and 2016.
Zhou J, Vallejo J, Kluetz P, Pazdur R, Kim T, Keegan P, Farrell A, Beaver JA, Sridhara R.
J Natl Cancer Inst. 2018 Aug 4. doi: 10.1093/jnci/djy130. [Epub ahead of print]
Metastasis-free Survival - A New End Point in Prostate Cancer Trials.
Beaver JA, Kluetz PG, Pazdur R.
N Engl J Med. 2018 Jun 28;378(26):2458-2460. doi: 10.1056/NEJMp1805966. No abstract available.
Informing the Tolerability of Cancer Treatments Using Patient-Reported Outcome Measures: Summary of an FDA and Critical Path Institute Workshop.
Kluetz PG, Kanapuru B, Lemery S, Johnson LL, Fiero MH, Arscott K, Barbachano Y, Basch E, Campbell M, Cappelleri JC, Cella D, Cleeland C, Coens C, Daniels S, Denlinger CS, Fairclough DL, Hillard JR, Minasian L, Mitchell SA, O'Connor D, Patel S, Rubin EH, Ryden A, Soltys K, Sridhara R, Thanarajasingam G, Velikova G, Coons SJ.
Value Health. 2018 Jun;21(6):742-747. doi: 10.1016/j.jval.2017.09.009. Epub 2017 Nov 7.
Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada.
Kluetz PG, O'Connor DJ, Soltys K.
Lancet Oncol. 2018 May;19(5):e267-e274. doi: 10.1016/S1470-2045(18)30097-4. Review.
Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension.
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT; the SPIRIT-PRO Group, Hunn A, Bottomley A, Regnault A, Chan AW, Ells C, O'Connor D, Revicki D, Patrick D, Altman D, Basch E, Velikova G, Price G, Draper H, Blazeby J, Scott J, Coast J, Norquist J, Brown J, Haywood K, Johnson LL, Campbell L, Frank L, von Hildebrand M, Brundage M, Palmer M, Kluetz P, Stephens R, Golub RM, Mitchell S, Groves T.
JAMA. 2018 Feb 6;319(5):483-494. doi: 10.1001/jama.2017.21903.
Use of PRO Measures to Inform Tolerability in Oncology Trials: Implications for Clinical Review, IND Safety Reporting, and Clinical Site Inspections.
Kim J, Singh H, Ayalew K, Borror K, Campbell M, Johnson LL, Karesh A, Khin NA, Less JR, Menikoff J, Minasian L, Mitchell SA, Papadopoulos EJ, Piekarz RL, Prohaska KA, Thompson S, Sridhara R, Pazdur R, Kluetz PG.
Clin Cancer Res. 2018 Apr 15;24(8):1780-1784. doi: 10.1158/1078-0432.CCR-17-2555. Epub 2017 Dec 13.
Commentary on Heath et al.
Kwitkowski V, Daniels S, Reaman G, Farrell A, Kluetz P.
Clin Trials. 2017 Dec;14(6):572-574. doi: 10.1177/1740774517723308. Epub 2017 Aug 2. No abstract available.
Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
Kluetz PG, Chingos DT, Basch EM, Mitchell SA.
Am Soc Clin Oncol Educ Book. 2016;35:67-73. doi: 10.14694/EDBK_159514. Review.
Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials-Response.
Kluetz PG, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, Chen WH, Sridhara R, Farrell AT, Keegan P, Kim G, Pazdur R.
Clin Cancer Res. 2016 Nov 15;22(22):5618. doi: 10.1158/1078-0432.CCR-16-2140. No abstract available.
Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards.
Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Dueck AC, Devlin N, Flechtner HH, Gotay C, Greimel E, Griebsch I, Groenvold M, Hamel JF, King M, Kluetz PG, Koller M, Malone DC, Martinelli F, Mitchell SA, Moinpour CM, Musoro J, O'Connor D, Oliver K, Piault-Louis E, Piccart M, Pimentel FL, Quinten C, Reijneveld JC, Schürmann C, Smith AW, Soltys KM, Taphoorn MJ, Velikova G, Coens C; Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) consortium.
Lancet Oncol. 2016 Nov;17(11):e510-e514. doi: 10.1016/S1470-2045(16)30510-1. Epub 2016 Oct 18. Review.
Looking to the future in an unprecedented time for cancer drug development.
Kluetz PG, Pazdur R.
Semin Oncol. 2016 Feb;43(1):2-3. doi: 10.1053/j.seminoncol.2016.01.001. Epub 2016 Jan 15.
Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials: Symptomatic Adverse Events, Physical Function, and Disease-Related Symptoms.
Kluetz PG, Slagle A, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, Chen WH, Sridhara R, Farrell AT, Keegan P, Kim G, Pazdur R.
Clin Cancer Res. 2016 Apr 1;22(7):1553-8. doi: 10.1158/1078-0432.CCR-15-2035. Epub 2016 Jan 12.
Patient-Reported Outcomes in Cancer Drug Development and US Regulatory Review: Perspectives From Industry, the Food and Drug Administration, and the Patient.
Basch E, Geoghegan C, Coons SJ, Gnanasakthy A, Slagle AF, Papadopoulos EJ, Kluetz PG.
JAMA Oncol. 2015 Jun;1(3):375-9. doi: 10.1001/jamaoncol.2015.0530. Review.
Pain palliation measurement in cancer clinical trials: the US Food and Drug Administration perspective.
Basch E, Trentacosti AM, Burke LB, Kwitkowski V, Kane RC, Autio KA, Papadopoulos E, Stansbury JP, Kluetz PG, Smith H, Justice R, Pazdur R.
Cancer. 2014 Mar 1;120(5):761-7. doi: 10.1002/cncr.28470. Epub 2013 Dec 5.