Hyland's Homeopathic Teething Tablets: Questions and Answers
What action did the FDA take?
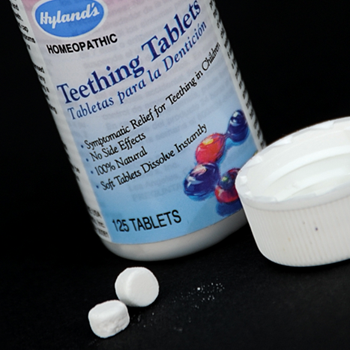
On October 23, 2010, the U.S. Food and Drug Administration (FDA) warned consumers to stop using and discard Hyland’s Teething Tablets; and the manufacturer recalled the product.
Why did the FDA take this action?
The FDA issued this warning because the use of Hyland’s Teething Tablets may pose a risk to children. The FDA’s analysis and testing identified some Hyland’s Teething Tablets that contained varying amounts of belladonna, a potentially toxic ingredient. The FDA has received reports of serious adverse events in children taking this product that are consistent with belladonna toxicity. An FDA inspection at the manufacturer’s facility indicated substandard control of the manufacturing operation.
The FDA has also received reports of children who consumed more tablets than recommended, because the containers did not have child resistant caps.
What product was affected by this warning?
The FDA’s warning affected all lots of Hyland’s Teething Tablets. At the time of this warning, the product was widely sold in pharmacies and other retail stores, and continues to be marketed online as an over-the-counter (OTC) homeopathic drug intended to provide temporary relief of symptoms related to teething in children.
What is belladonna?
Belladonna is commonly known as Deadly Nightshade. It is a plant whose leaves and berries are extremely toxic. Belladonna has been used as both a poison and a medicine throughout history.
What are symptoms of belladonna toxicity or overdose?
Belladonna alkaloids have anticholinergic effects. Classic signs of anticholinergic toxicity include fast heart rate, increased body temperature, dry skin and dry mouth, skin flushing, constipation, decreased urination, agitation, disorientation, hallucinations, and dilated pupils. Drowsiness may also be seen in infants.
Are Hyland’s Teething Tablets approved by the FDA?
The FDA has not evaluated Hyland’s Teething Tablets for safety or efficacy, and is not aware of any proven clinical benefit offered by this product.
What should consumers do if they experience harm related to these products?
The FDA recommends that consumers contact their health care professional if their child experiences symptoms after taking Hyland’s Teething Tablets. Symptoms include a depressed level of consciousness, seizure, difficulty or slowed breathing, lethargy, sleepiness, muscle weakness, skin flushing, constipation, difficulty urinating, or agitation.
Health care professionals and consumers should report side effects from use of Hyland’s teething tablets to the FDA through the MedWatch program, by phone at 1-800-332-1088, or online at http://www.fda.gov/medwatch/index.html
What further steps has the FDA taken?
On September 30, 2016 the FDA issued another advisory warning consumers to stop using and to discard or return the Hyland’s Teething Tablet product; and the agency’s investigation of the product and the firm’s manufacturing operations is ongoing.
