Infant Formula Supply
Information and resources from the FDA and government partners

In the coming weeks and months, you should see more infant formula on store shelves as the U.S. Food and Drug Administration helps to increase supply to address production and distribution issues. Some of the products will be new to the U.S. market, and therefore new to you. It is important to talk with your child’s health care provider for recommendations on changing feeding practices as you consider your options.
The FDA and our government partners have created information resources to help you make decisions for feeding your baby until infant formula supplies return to normal.
FDA and HHS Information Resources
The FDA webpage, Infant Formula Information and Ongoing FDA Efforts to Increase Supply, includes information about the infant formula products allowed in the U.S., tips for preparing the formula, and the latest infant formula news from the FDA.
And the U.S. Department of Health and Human Service webpage, Information for Families During the Formula Shortage, has a wide range of information in multiple languages. It includes advice from physicians and experts on trying new formulas and safe substitution.
Safely Increasing the Supply of Infant Formula
The FDA is aware parents and caregivers have experienced challenges in consistently locating infant formula on store shelves. Production and distribution issues have led to reduced supplies in some parts of the country.
To increase the supply of infant formula in the U.S., the FDA in mid-May set up a streamlined process so that companies in the U.S. and in other countries that don’t normally sell their infant formula in the U.S. can request to do so.
To help ensure safety of the infant formula distributed in the U.S., the information FDA is reviewing includes:
- The infant formula’s nutritional composition and ingredients.
- Microbiological and nutrient testing for the finished product.
- Facility inspection history.
- Manufacturing procedures and controls.
- Quality control procedures.
The FDA also will ensure any new infant formula products have labeling information about allergens and directions for preparing formula.
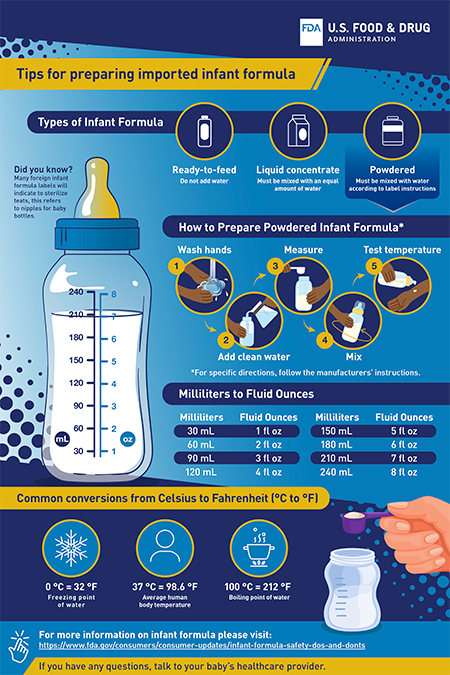
Tips for Preparing Imported Formula
Because some of the formula allowed for distribution comes from other countries, the labels and directions may have terms you are not used to seeing.
To help you safely prepare imported infant formula, the FDA created a tip sheet. It shows conversion measurements from milliliters to fluid ounces and temperatures from Celsius to Fahrenheit (◦C to ◦F).
Tips for Preparing Imported Infant Formula (PDF)
Identifying New Infant Formula Products Reviewed by the FDA
The streamlined process the FDA initiated to increase infant formula supply applies to companies outside the U.S. and to some companies in the U.S. as well. For example, a U.S. company that makes infant formula solely for export could request to sell their product here.
The FDA developed a webpage listing the infant formula allowed to be distributed in the U.S. under the streamlined process, known as enforcement discretion.
The webpage covers regular infant formula and specialty formulas for infants with special medical needs. Information is being updated regularly.
Avoiding Counterfeit Infant Formula Products
If you use an online marketplace to buy infant formula that’s made outside the U.S., be cautious—the product has the potential to be counterfeit. The FDA has information on what counterfeit formulas are and how you can avoid buying them.