Powdered Infant Formula Recall: What to Know
Do not use recalled Similac, Alimentum and EleCare powdered infant formulas produced in Sturgis, Michigan.

If you use powdered infant formula, be aware certain Similac, Alimentum and EleCare products have been recalled and should not be used.
The U.S. Food and Drug Administration (FDA) is investigating consumer complaints of bacterial infections in four infants who consumed powdered infant formula produced in Abbott Nutrition’s facility in Sturgis, Michigan. All four infants had to be hospitalized and the bacterial infection may have contributed to death in two patients.
The FDA has published a full list of recalled brands. Recalled products should no longer be available for sale. But if you have these products in your home, check the lot code on the bottom of the package to determine if they are included in the recall.
The FDA also is providing additional information for parents and caregivers of infants receiving medical specialty infant formula and individuals using certain medical foods.
Because infant formula is the only source of nutrition for many newborns and infants, the FDA understands and shares the concerns parents and caregivers may have.
Here’s information to help you as we continue our investigation.
What powdered infant formula products have been recalled?
Abbott Nutrition has recalled certain powdered infant formula products produced at its Sturgis, Michigan facility. Products from that facility can be found across the U.S. and some were exported to other countries. Here’s how you can tell if you have any of those products.
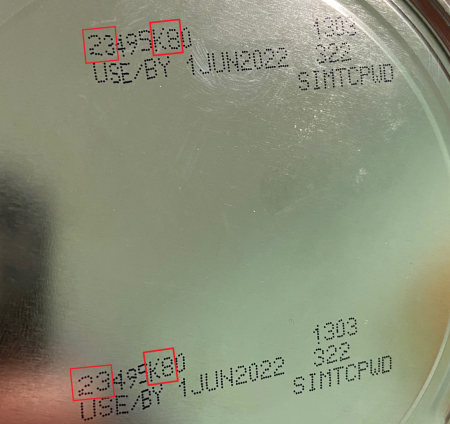
The FDA recommends consumers look at the lot code, a multidigit number on the bottom of a container of Similac, Alimentum and EleCare powdered infant formula and do not use if:
- the first two digits of the code are 22 through 37; and
- the code on the container contains K8, SH or Z2; and
- the expiration date is 4-1-2022 (APR 2022) or later.
In addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code of 27032K80 (can) / 27032K800 (case).
You can also enter your product lot code on the company’s website to check if it is part of the recall. Please see the images below for a closer look at the identifying information.
Powdered Abbott products that don’t have the code and expiration noted above are not included in the recall. Liquid formula products are not subject to the recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
What infections have been reported and what symptoms should I look for?
All four cases involve Cronobacter sakazakii infection.
- Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine). Cronobacter infections are rare but are especially high risk for newborns.
- Symptoms related to Cronobacter infection include: poor feeding, irritability, temperature changes, jaundice, grunting breaths, or abnormal body movements.
- If your infant is experiencing symptoms related to Cronobacter infection, contact your child’s health care provider to report his or her symptoms and receive immediate care.
When and where were the illnesses?
Illnesses occurred in Minnesota, Ohio, and Texas between September 6, 2021 and January 4, 2022.
I’m having a hard time finding formula. What is the FDA doing to help?
We are aware the recall has created new concerns about the availability of certain types of infant formula, particularly given the overall strains on supply chains experienced during the COVID-19 pandemic.
The FDA continues to take several significant actions to help increase the current supply of infant formula in the U.S. In fact, other infant formula manufacturers are meeting or exceeding capacity levels to meet current demand.
The FDA is working with Abbott Nutrition to better assess the impacts of the recall and understand the production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott on safe resumption of production at the Sturgis, Michigan facility. As Abbott Nutrition was initiating its recall, the FDA intensified outreach to other infant formula manufacturers to inquire about their capacity and potential impacts. We will continue discussion with Abbott Nutrition and other infant formula manufacturers and consider all tools available to support the supply of infant formula products.
Are homemade formulas an alternative?
No. The FDA advises parents and caregivers not to make or feed homemade formula to infants. Homemade infant formula recipes have not been evaluated by the FDA and may lack nutrients vital to an infant’s growth.
What should I know about medical specialty infant formula and certain medical foods?
The Abbott Nutrition facility that produces recalled infant formulas also produces metabolic and other medical specialty infant formulas for infants with inborn errors of metabolism and other medical needs, as well as medical foods. These products, with the exception of one lot of Abbott Similac PM 60/40, have not been recalled because the FDA has determined that the risk of not having these specialty products available would significantly worsen underlying medical conditions. For many of these patients, the risk of life-threatening adverse events from restricted access to these critically needed products is likely greater than the risk from consuming products that have been produced at the facility.
The FDA wants to be sure that parents and caregivers who use these specialty products are aware that there may be some risk of Cronobacter contamination. If possible, parents and caregivers should work with their medical provider to consider whether comparable products may be appropriate. If comparable alternative products are not available or appropriate, parents and caregivers should take extra care to follow the CDC’s updated advice for parents on how to reduce the risk of Cronobacter contamination of formula during preparation of powdered product, whether that contamination comes from the product itself or from other contamination sources in the home.
Examples of medical and specialty products include Glutarex-1, Glutarex-2, Cyclinex-1, Cyclinex-2, Hominex-1, Hominex-2, I-Valex-1, I-Valex-2, Ketonex-1, Ketonex-2, Phenex-1, Phenex-2, Phenex-2 Vanilla, Pro-Phree, Propimex-1, Propimex-2, ProViMin, Calcilo XD, Tyrex-1, Tyrex-2, and Similac PM 60/40.
It is important to note that these specialty infant formulas and medical foods are not sold in traditional retail stores. These products often require a prescription and are sold through specialty pharmacies and other specialty distribution channels such as medical product suppliers.
Parents and caregivers of infants and children using these products should contact their child’s health care providers if they have questions about the use of these products.
Additionally, the FDA has informed Abbott Nutrition that the agency has no objection to the company immediately releasing product to individuals needing urgent, life-sustaining supplies of the specialty and metabolic formulas listed in the link below on a case-by-case basis. Abbott has confirmed with the FDA that the company will consider release of these products on a case-by-case basis, depending on product availability and the severity of the individual’s need. Patients and caregivers seeking access to these products should contact Abbott directly to request that a product be made available to them by calling 1-800-881-0876. For more information on those products, please see FDA Investigation of Cronobacter Infections: Powdered Infant Formula (February 2022).
What else should I know?
Parents and caregivers also should never dilute infant formula. Consumers also should avoid buying formula online that comes from outside the U.S., as it has the potential to be counterfeit.
If your regular formula is not available, contact your child’s health care provider for recommendations on changing feeding practices.
If you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance.
Additional Resources:
- Infant Formula: Safety Do’s and Don’ts, FDA Consumer Update
- FDA Investigation of Cronobacter Infections: Powdered Infant Formula (February 2022), FDA webpage
- Cronobacter Infection and Infants, CDC webpage