Coronavirus Treatment Acceleration Program (CTAP)
On this page
- What is CTAP? and other Frequently Asked Questions
- FDA Voices Articles on CTAP
- CTAP Dashboard
- Key Resources on Therapeutic Development
- For Researchers and Developers of Therapeutics
- Contact Information for Sponsors
- For Patients and Consumers
- Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Partnership
- CDER and CBER Emergency Use Authorization Transparency
What is CTAP?
FDA has created a special emergency program for possible coronavirus therapies, the Coronavirus Treatment Acceleration Program (CTAP). The program uses every available method to move new treatments to patients as quickly as possible, while at the same time finding out whether they are helpful or harmful. We continue to support clinical trials that are testing new treatments for COVID so that we gain valuable knowledge about their safety and effectiveness. Please see our Frequently Asked Questions page for more information on the program.
FDA Voices Articles on CTAP
On April 20, 2020, Commissioner Hahn, CBER Director Marks, and CDER Director Woodcock explain FDA’s strategic approach to COVID-19 therapeutic development via a blog post, "CTAP: The Path Forward."
On July 14, 2020, Commissioner Hahn, Acting CDER Director Cavazzoni and CBER Director Marks explain how we built and manage CTAP operations via a blog post, “CTAP: Behind the Scenes.”
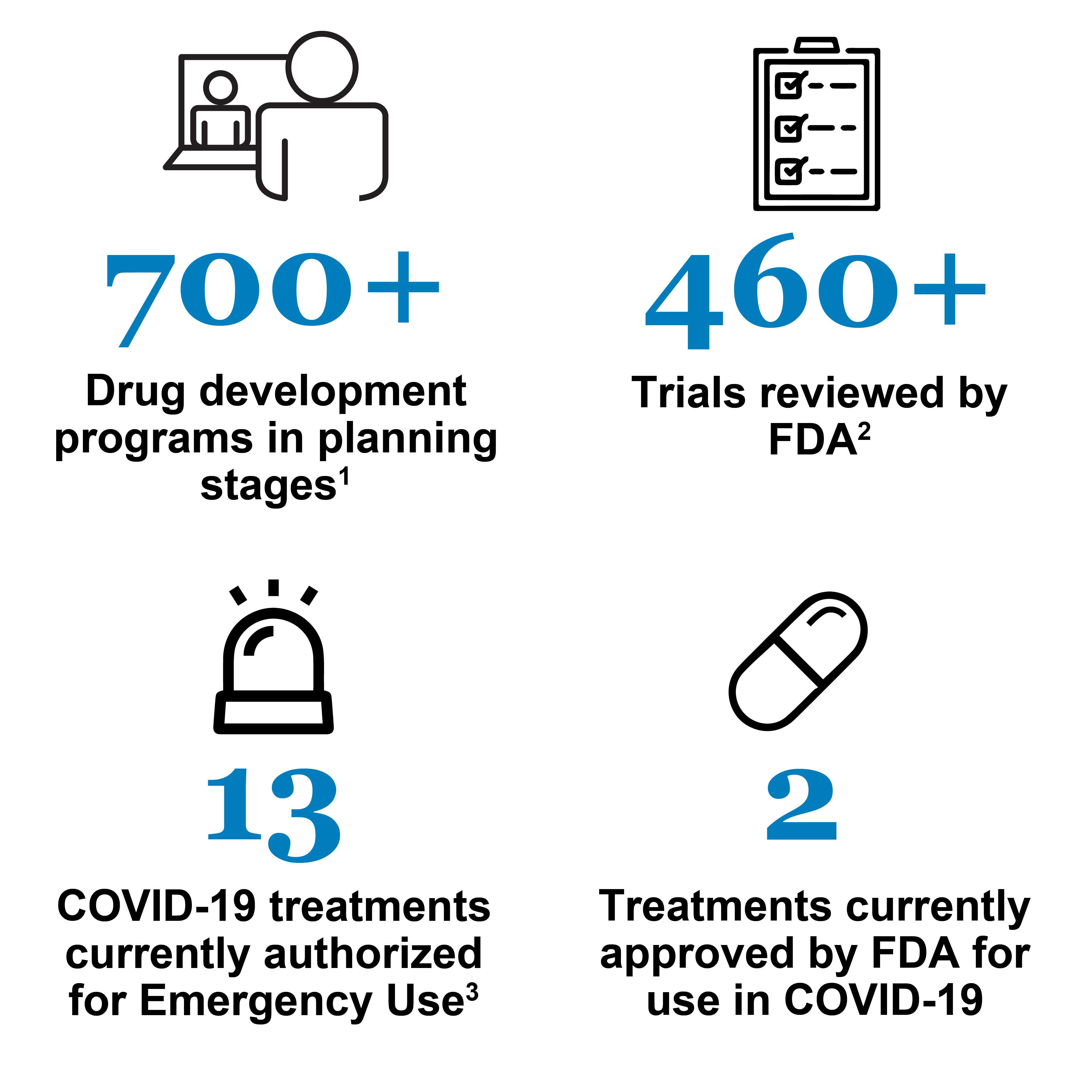
CTAP Dashboard
This dashboard provides a snapshot of development of potential COVID-19 therapeutics. Given the urgent nature of the pandemic and the number of companies and researchers developing COVID-19 related therapies, the following numbers may change frequently. FDA will update these numbers monthly. As of May 31, 2022, the snapshot is:
2Safe to proceed INDs. These only include ongoing trials (e.g., withdrawn or terminated trials are not included). Ongoing trials reviewed by FDA include antiviral drugs, immunomodulators, neutralizing antibody therapies, cell therapies and gene therapies; vaccines are excluded. These trials fall into two categories: early stage and late stage. Early stage trials (phase 0, 1 and 1/2) test safety and sometimes dosing. They rarely provide sufficient evidence to support Emergency Use Authorization or approval. Late stage trials (phase 2, 2/3, 3, and 4) assess safety and establish whether the treatment is effective. They may generate sufficient evidence to support the statutory standards for Emergency Use Authorization or approval. For additional information regarding the different phases of clinical trials, please visit: What Are the Different Types of Clinical Research?
3Please see the Emergency Use Authorization webpage for more details. This number includes 1 EUA authorizing both medical devices and a drug for emergency use.
COVID-19 related therapies include antiviral drugs, immunomodulators, neutralizing antibody therapies, cell therapies and gene therapies. The diversity of therapeutic approaches being investigated is important because it rapidly expands our understanding of the effect of different categories of potential treatments.
Antiviral drugs keep viruses from multiplying and are used to treat many viral infections (such as HIV, Herpes, Hepatitis C, and influenza).
Immunomodulators are aimed at tamping down the body’s own immune reaction to the virus, in cases where the body’s reaction basically goes overboard and starts attacking the patient’s own organs.
Neutralizing antibody therapies may help individuals fight the virus and include manufactured antibodies, animal-sourced antibody therapies, and blood-derived products such as convalescent plasma and hyperimmune globulin, which contain antibodies taken from people who have previously had COVID-19.
Cell therapy products include cellular immunotherapies and other types of both autologous and allogeneic cells, such as stem cells, and related products.
Gene therapy products seek to modify or manipulate the expression of a gene or to alter the biological properties of living cells for therapeutic use.
Key Resources on Therapeutic Development
We understand companies, researchers, patients and consumers need timely information on therapeutic development for COVID-19. Below are key resources for researchers and therapeutic developers, and patients and consumers.
For Researchers and Developers of Therapeutics
FDA is accelerating the development and publication of guidances and other information for industry on developing COVID-19-related treatments. Key references include:
- Information and guidance on how to efficiently engage with FDA and expedite clinical trial initiation may be found at: Drug Development Inquiries for Drugs to Address the COVID-19 Public Health Emergency
- General advice concerning the development of COVID-19 treatments may be found at: COVID-19: Developing Drugs and Biological Products for Treatment or Prevention
- Recommendations on the generation of data to support an emergency use authorization (EUA) for monoclonal antibody products targeting SARS-CoV-2 can be found at: Development of Monoclonal Antibody Products Targeting SARS-CoV-2, Including Addressing the Impact of Emerging Variants, During the COVID-19 Public Health Emergency
- General advice concerning pre-IND meeting request content for COVID-19 treatments is provided at: COVID-19 Public Health Emergency: General Considerations for Pre-IND Meeting Requests for COVID-19 Related Drugs and Biological Products
- Information on expediting quality assessments for products to treat COVID-19 patients and transferring manufacturing to new or alternative sites to avoid supply disruptions may be found at: COVID-19 Manufacturing, Supply Chain, and Drug Inspections
- More information about the availability of COVID-19 treatments under an Emergency Use Authorization may be found at: FDA’s Emergency Use Authorization (EUA)
- Consolidated resources relevant to drug manufacturers making products for the U.S. market may be found at: Developing and Manufacturing Drugs Including Biologics
Contact Information for Sponsors
- Sponsors of CDER regulated therapeutics should send COVID-19 product development inquiries to COVID19-productdevelopment@fda.hhs.gov
- Sponsors of CBER regulated therapeutics should send COVID-19 product development inquiries to CBERProductJurisdiction@fda.hhs.gov. Additional information about CBER-Regulated Therapeutics and CTAP can be found at Coronavirus (COVID-19) | CBER-Regulated Biologics
- Sponsors who are unsure of whether their drug is CDER- or CBER-regulated should make initial contact for COVID-19 drug development by contacting FDA at COVID19-productdevelopment@fda.hhs.gov
- Medical devices do not fall within the CTAP program. Device sponsors should contact CDRH directly at CDRH-EUA-Templates@fda.hhs.gov for in vitro diagnostics (IVDs) and CDRH-NonDiagnosticEUA-Templates@fda.hhs.gov for non-IVD medical devices
- As a general matter, if your product is already assigned to CBER or CDRH or you have been in contact with Center review staff regarding your product, submission of additional inquiries or materials to the CTAP mailbox are not necessary and may result in unnecessary delays in processing your information
For Patients and Consumers
CTAP’s primary goal is to accelerate the development of therapeutics for patients and consumers. Here are a number of resources that may be of interest to patients and consumers:
- More information on how FDA ensures the safety of patients in COVID-19 clinical trials may be found at: Ensuring the Safety of Patients in Clinical Trials Studying Investigational New Drugs to Prevent or Treat COVID-19
- Clearly understandable explanations of key technical terms are at: Understanding the Regulatory Terminology of Potential Preventions and Treatments for COVID-19
- Answers to frequently asked questions about therapies for COVID-19 and FDA’s related work can be found at: FDA’s Frequently Asked Questions on COVID-19
- If you have fully recovered from COVID-19, you may be able to help patients currently fighting the infection by donating your plasma. Information can be found here: Donate COVID-19 Plasma
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Partnership
Another critical initiative is the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, coordinated by the Foundation for the National Institutes of Health (FNIH). ACTIV involves a collaboration among government and industry partners, including FDA, to prioritize vaccine and therapeutic candidates, streamline clinical trials, and rapidly expand the clinical research resources focused on developing therapies for the COVID-19 pandemic. Among other things, ACTIV government and industry partners provide subject matter expertise and/or funding to identify, prioritize and facilitate the entry of some of the most promising candidates into clinical trials.
CTAP plays an important role in these efforts by providing FDA subject matter expertise for ACTIV initiatives, including for clinical trial design and conduct and relevant FDA regulatory standards for therapeutics. Under the CTAP program, FDA can better ensure that critical focus is placed on reviewing those therapies prioritized by the ACTIV partnership. The involvement of FDA in the ACTIV partnership will also help ensure these reviews are more efficient, particularly in evaluating proposed pre-clinical and clinical studies that received ACTIV input. This more comprehensive and cooperative approach involving key partners can help ensure that safe and effective therapies for COVID are available more quickly for patients. However, it should be noted that FDA’s regulatory functions are distinct from its contribution of technical advice to other US government programs. The FDA will evaluate each product submitted for authorization or approval based on the applicable legal and regulatory requirements and on the bases of the best available scientific and clinical evidence.
CDER and CBER Emergency Use Authorization Transparency
We believe that transparency about CDER’s and CBER’s review of the scientific information supporting their recommendations to issue Emergency Use Authorizations (EUAs) for drugs or biological products promotes public confidence in FDA’s scientific review process and ultimately in using the authorized products appropriately.
Therefore, our goal is to be as transparent as possible about the scientific basis for recommending that a drug or biological product be authorized for emergency use under section 564 of the Federal Food, Drug and Cosmetic Act (21 U.S.C. 360bbb-3) or be revised or revoked.
- When a CDER-regulated or CBER-regulated product is authorized for emergency use, we intend to the extent appropriate and permitted by law to make public the Center’s review of the scientific data and information supporting our recommendation to issue the EUA.
- When an EUA is revised, we also intend to make public the Center’s review of the scientific data and information supporting our recommendations to revise the EUA.
Our goal is to disclose information from our EUA review documents as appropriate and consistent with our longstanding practice of posting scientific reviews after new drug and biological product approvals. We may redact certain information that is exempt from disclosure under the Freedom of Information Act (FOIA), 5 U.S.C. sec. 552, such as trade secrets or other information identified by the EUA requestors that is exempt under FOIA. The redacted information may vary depending on the type of data contained in the reviews and whether the requestor consents to the release of information that is exempt under FOIA.