Drug Trial Snapshot: ELZONRIS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ELZONRIS Package Insert for complete information.
ELZONRIS (tagraxofusp-erzx)
el zon’ ris
Stemline Therapeutics, Inc.
Approval date:December 21, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ELZONRIS is a drug for treatment of patients 2 years and older who have a blastic plasmacytoid dendritic cell neoplasm (BPDCN).
BPDCN is a rare, rapidly progressing cancer of the bone marrow and blood that can affect multiple organs, including the skin, lymph nodes, spleen, and liver.
How is this drug used?
ELZONRIS is injected into a vein (intravenous) by a healthcare provider once daily on days 1 through 5 of a 21-day cycle. It takes about 15 minutes to receive an ELZONRIS infusion, and patients are hospitalized for the duration of the first cycle.
The dose depends on a patient’s weight.
What are the benefits of this drug?
Out of 13 patients who had never been treated for BPDCN, 7 patients (54%) achieved no evidence of disease (complete remission or CR) or no evidence of disease with some skin changes not indicative of active disease (CRc) after treatment with ELZONRIS.
Out of 15 patients with worsening or resistant BPDCN, 2 patients experienced no evidence of disease (CR) or no evidence of disease with some skin changes not indicative of active disease (CRc) after treatment with ELZONRIS.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table and figure below summarize efficacy results for the evaluated patients in the trial. The primary outcome was the rate of complete response or clinical complete response (CR/CRc).
Table 2. Efficacy Measure in Patients with Treatment-Naive BPDCN
| Parameter | N=13 |
|---|---|
| CR/CRc* Rate, N (%) (95% CI) |
7 (53.8) |
* CRc is defined as complete response with residual skin abnormality not indicative of active disease.
ELZONRIS Prescribing Information
Figure 4. Efficacy Measure in Patients with Relapsed or Refractory BPDCN
| In the 15 patients with relapsed/refractory BPDCN, one patient achieved a CR and one patient achieved a CRc. |
Adapted from ELZONRIS Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The number of patients completing the trial was too small (28 patients) to determine how well the drug worked in sex, race, and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The number of patients completing the trial who never had previous treatment for BPDCN or had worsening/resistant BPDCN was too small (28 patients) to determine differences in sex, race, and age subgroups.
What are the possible side effects?
ELZONRIS may cause a serious and life-threatening side effect called capillary leak syndrome (leakage of fluid and proteins out of tiny blood vessels into surrounding tissue). Other serious side effects include allergic reactions, and abnormal liver tests.
The most common side effects are capillary leak syndrome, nausea, tiredness, swelling of lower limbs, fever, and weight increase.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with BPDCN in the trial (safety population).
Table 3. Adverse Reactions in ≥ 10% of Patients Receiving 12 mcg/kg of ELZONRIS
|
N=94 |
||
|---|---|---|
|
All Grades |
Grade ≥ 3 |
|
|
Vascular disorders |
||
|
Capillary leak syndrome1 |
55 |
9 |
|
Hypotension |
29 |
9 |
|
Hypertension |
15 |
6 |
|
Gastrointestinal Disorders |
||
|
Nausea |
49 |
0 |
|
Constipation |
23 |
0 |
|
Vomiting |
21 |
0 |
|
Diarrhea |
20 |
0 |
|
General disorders and administration site conditions |
||
|
Fatigue |
45 |
7 |
|
Peripheral edema |
43 |
1 |
|
Pyrexia |
43 |
0 |
|
Chills |
29 |
1 |
|
Investigations |
||
|
Weight increase |
31 |
0 |
|
Nervous system disorders |
||
|
Headache |
29 |
0 |
|
Dizziness |
20 |
0 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
24 |
0 |
|
Blood and lymphatic system disorders |
||
|
Febrile neutropenia |
20 |
18 |
|
Musculoskeletal and connective tissue disorders |
||
|
Back pain |
20 |
2 |
|
Pain in extremity |
10 |
2 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Dyspnea |
19 |
2 |
|
Cough |
14 |
0 |
|
Epistaxis |
14 |
1 |
|
Oropharyngeal pain |
12 |
0 |
|
Psychiatric disorders |
||
|
Insomnia |
17 |
0 |
|
Anxiety |
15 |
0 |
|
Confusional state |
11 |
0 |
|
Cardiac disorders |
||
|
Tachycardia |
17 |
0 |
|
Skin and subcutaneous tissue disorders |
||
|
Petechiae |
10 |
0 |
|
Pruritus |
10 |
0 |
|
Renal and urinary disorders |
||
|
Hematuria |
10 |
0 |
1Capillary Leak Syndrome defined as any event reported as Capillary Leak syndrome (CLS) during treatment with ELZONRIS or the occurrence of at least 2 of the following CLS manifestations within 7 days of each other : hypoalbuminemia (including albumin levels less than 3.0 g/d), edema (including weight increase of 5kg or more), hypotension (including systolic blood pressure lees than 90 mmHg).
ELZONRIS Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among other races could not be determined.
- Age: The occurrence of altered mental state was higher in patients 75 years of age and older.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize selected adverse events that occurred in the clinical trial by subgroups.
Table 4. Subgroup Analysis of Adverse Events by Sex
|
Adverse Event |
ELZONRIS (N=94) |
|
|---|---|---|
|
Male (N=72) |
Female (N=22) |
|
|
Increased ALT |
62 (86) |
15 (68) |
|
Increased AST |
58 (81) |
16 (73) |
Clinical Trial Data
Table 5. Subgroup Analysis of Adverse Events by Race
|
Adverse Event |
ELZONRIS (N=94) |
||||
|---|---|---|---|---|---|
|
White (N=85) |
Black or African American (N=4) |
Asian (N=3) |
Other (N=2) |
||
|
Increased ALT |
70 (82) |
4 (100) |
1 (33) |
2(100) |
|
|
Increased AST |
67 (79) |
4 (100) |
1 (33) |
2 (100) |
|
Clinical Trial Data
Table 6. Subgroup Analysis of Adverse Events by Age
|
Adverse Event |
ELZONRIS (N=94) |
|
|---|---|---|
|
<75 years (N=72) |
> 75 years (N=22) |
|
|
Increased ALT |
60 (83) |
17 (77) |
|
Increased AST |
56 (78) |
18 (82) |
|
Altered Mental Status |
5 (7) |
10 (45) |
Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved ELZONRIS based primarily on evidence from one clinical trial (NCT02113982/Trial 0114) of 94 patients with cancers that arise from cells that form white blood cells including blastic plasmacytoid dendritic cell neoplasm (BPDCN). The trial was conducted in the United States.
Demographics of the patients who provided data for evaluation of benefits (efficacy population) are presented in Table 8, under the MORE INFO section. All patients had BPDCN some have never been treated for BPCN before, and some had worsening BPDCN, or BPDCN that did not respond to previous treatment.
The population that provided data for side effects of ELZONRIS (safety population) is presented below. This population includes all patients who received ELZONRIS (at the recommended dose) and had either BPDCN or other types of white cell blood cancer.
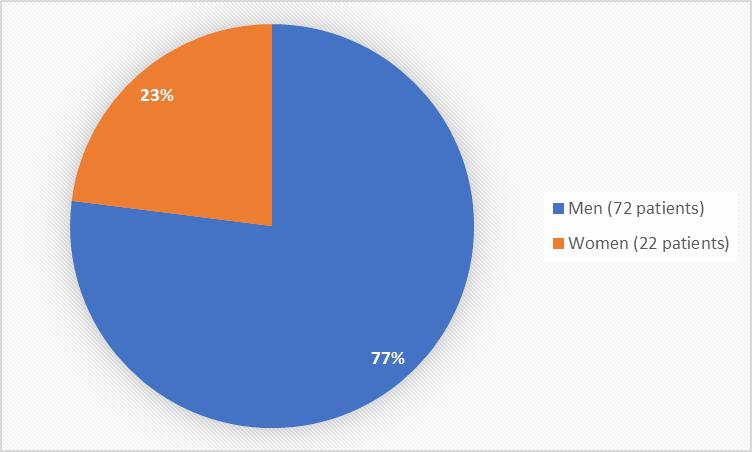
Figure 1 summarizes how many men and women were in the clinical trial used to evaluate safety.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
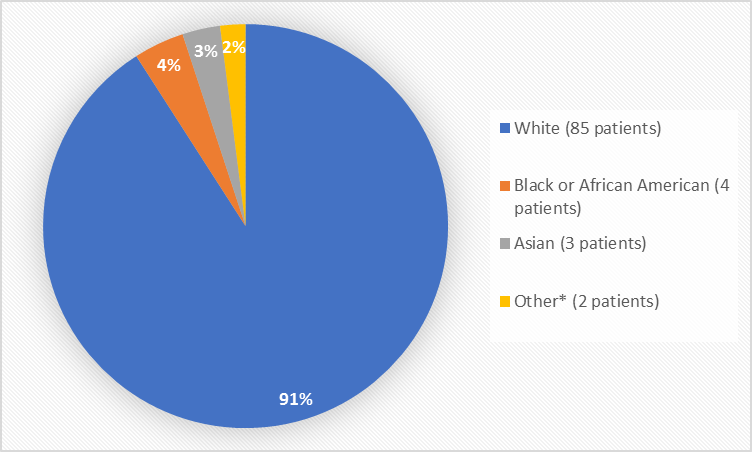
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial used to evaluate safety.
Figure 2. Baseline Demographics by Race (safety population)
*Other includes American Indians
Clinical Trial Data
Table 1. Demographics of Safety Trial by Race (safety population)
|
Race |
Number of Patients |
Percentage of Patients |
|---|---|---|
|
White |
85 |
90% |
|
Black or African American |
4 |
4% |
|
Asian |
3 |
3% |
|
American Indian |
1 |
1% |
|
Other |
1 |
1% |
Clincal Trial Data
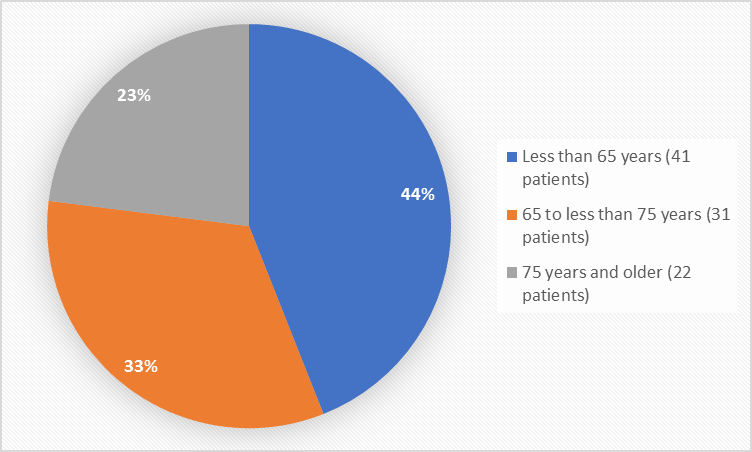
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Who participated in the trials?
The table below summarizes the demographics of the safety population.
Table 7. Demographics of the Safety Population
|
Demographic Characteristics |
ELZONRIS N = 94 |
|---|---|
|
Sex |
|
|
Male |
72 (77) |
|
Female |
22 (23) |
|
Race |
|
|
White |
85 (90) |
|
Black or African American |
4 (4) |
|
Asian |
3 (3) |
|
American Indian or Alaska Native |
1 (1) |
|
Other |
1 (1) |
|
Age (years) |
|
|
18 to < 65 years |
41 (44) |
|
65 to < 75 years |
31 (33) |
|
> 75 years |
22 (23) |
|
Median (range) |
66.5 (21 – 87) |
|
Ethnicity |
|
|
Hispanic |
9 (10) |
|
Non-Hispanic |
85 (90) |
|
Region |
|
|
United States |
94 (100) |
Clinical Trial Data
The table below summarizes the demographics of the intent-to-treat (ITT) population.
Table 8. Demographics of the Intent-to-Treat Population (efficacy population)
|
Demographic Characteristic |
Treatment-Naïve BPDCN |
Relapsed or Refratory BPDCN |
|---|---|---|
|
Sex |
||
|
Men |
11 (85) |
13 (87) |
|
Women |
2(15) |
2 (13) |
|
Race |
||
|
White |
13 (100) |
13 (87) |
|
American Indian or Alaska Native |
0 (0) |
2 (13) |
|
Age (years) |
||
|
< 65 years |
5 (38) |
4 (27) |
|
> 65 years |
8 (62) |
11 (73) |
|
Median (range) |
65 (22-84) |
72 (44-80) |
|
Ethnicity |
||
|
Hispanic |
0 (0) |
0 (0) |
|
Not Hispanic |
13 (100) |
15(100) |
|
Region |
||
|
United States |
13 (100) |
15 (100) |
Clinical Trial Data
How were the trials designed?
The trial enrolled adult patients who were 18 years and older with cancers that arise from cells that form blood cells including blastic plasmacytoid dendritic cell neoplasm (BPDCN). Patients with BPDCN had never been treated previously or their BPDCN was worsening or did not respond to treatment.
The trial consisted of four stages. Stage 1 was to determine the maximum dose of ELZONRIS that could be tolerated for use in Stages 2 through 4. Patients received increasing doses of ELZONRIS intravenously for 5 days of a 21-day cycle. Stage 2 evaluated patients with any type of BPDCN. Stage 3 only evaluated patients not previously treated for BPDCN. Stage 4 evaluated and treated seriously ill patients with BPDCN when no other treatments are available. Stage 4 is still ongoing. Patients enrolled in Stages 2 through 4 received the same dose of ELZONRIS intravenously for 5 days of a 21-day cycle.
The benefit of ELZONRIS was assessed by measuring how many patients reached complete response (no evidence of disease) with or without remaining skin abnormalities.
How were the trials designed?
The efficacy and safety of ELZONRIS were primarily evaluated in one open-label, single-arm clinical trial.
The trial enrolled adult patients who were 18 years and older with newly-diagnosed or relapsed/refractory myeloid malignancies including blastic plasmacytoid dendritic cell neoplasm (BPDCN). The trial consisted of four stages. Stage 1 was a dose escalation phase in any BPDCN and relapsed/refractory AML patients to determine the maximum tolerated dose (MTD) of ELZONRIS for use in Stages 2 through 4. Patients received 7, 9, and 12 µg/kg/day doses of ELZONRIS intravenously for 5 days of a 21-day cycle. Stage 2 was a dose expansion phase evaluating patients with any type of BPDCN. Stage 3 evaluated the pivotal cohort of patients not previously treated for BPDCN. Stage 4 evaluated the expanded access cohort and is still ongoing. Patients enrolled in Stages 2 through 4 received 12 µg/kg/day of ELZONRIS intravenously for 5 days of a 21-day cycle.
The primary efficacy endpoint was the CR (complete response) + CRc (clinical complete response with minimal residual skin abnormality) rate based on tumor response criteria for bone marrow, peripheral blood, skin, nodal masses, spleen, and liver.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION