Drug Trial Snapshot: Erleada
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ERLEADA Package Insert for complete information.
Erleada
(er lee’dah)
Janssen Biotech, Inc.
Approval date: February 14, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ERLEADA is a drug for the treatment of prostate cancer that has not spread to other parts of the body (non-metastatic) and no longer responds to a medical or surgical treatment that lowers testosterone (castration-resistant).
How is this drug used?
ERLEADA is a tablet. Four tablets (total of 240 mg) are taken once daily with or without food.
What are the benefits of this drug?
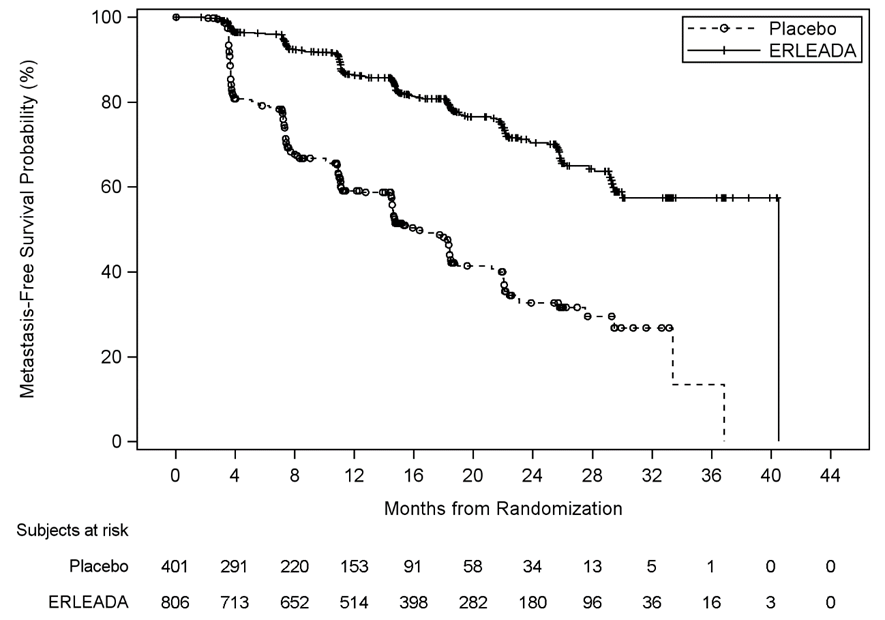
In the trial, ERLEADA increased the patients’ survival during which there was no spreading of the cancer (called metastasis-free survival). The median metastasis-free survival for patients taking ERLEADA was 40.5 months compared to 16.2 months for patients taking a placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary efficacy outcome measure of the clinical trial was metastasis-free survival (MFS). MFS was defined as the time from randomization to the time of first evidence of blinded independent central review (BICR)-confirmed distant metastasis, defined as new bone or soft tissue lesions or enlarged lymph nodes above the iliac bifurcation, or death due to any cause, whichever occurred first. Additional efficacy endpoints were time to metastasis (TTM), progression-free survival (PFS) time to symptomatic progression, and overall survival (OS).
The efficacy results are shown in Figure 4 and Table 2.
Figure 4. Kaplan-Meier Metastasis-Free Survival (MFS) Curve in ERLEADA Clinical Trial
ERLEADA Prescribing Information
Table 2: BICR-assessed Efficacy Results in ERLEADA Clinical Trial
| Endpoint | Number of Events (%) | Median [Months (95% CI)] | HR (95% CI) p-value (log-rank test)1 | ||
|---|---|---|---|---|---|
| ERLEADA+ADT (N=806) | Placebo+ADT (N=401) | ERLEADA+ADT | Placebo+ADT | ||
| Metastasis Free Survival | 184 (23%) | 194 (48%) | 40.51 (NE, NE) | 16.20 (14.59, 18.40) | 0.28 (0.23, 0.35) |
| Time to Metastasis | 175 (22%) | 191 (48%) | 40.51 (NE, NE) | 16.59 (14.59, 18.46) | 0.27 (0.22, 0.34) |
| Progression-Free Survival | 200 (25%) | 204 (51%) | 40.51 (NE, NE) | 14.72 (14.49, 18.37) | 0.29 (0.24, 0.36) |
NE=Not Estimable
1All analyses stratified by PSA doubling time, bone-sparing agent use, and locoregional disease status.
ERLEADA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All the patients were men since ERLEADA is for the treatment of prostate cancer.
- Race: ERLEADA worked similarly in White, Black or African American, and Asian patients.
- Age: ERLEADA worked similarly in patients below or above 65 years of age, including patients above 75 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below shows metastasis-free survival by subgroup. These exploratory analyses should be interpreted with caution.
Table 3. Metastasis Free Survival by Subgroups
| Number of Events/Total N (% of Patients with Event) | Median (months) | HR (95% CI)1 | |||
|---|---|---|---|---|---|
| ERLEADA | Placebo | ERLEADA | Placebo | ||
| Race | |||||
| White | 121/524 (23) | 143/276 (52) | 40.5 | 14.6 | 0.26 (0.21, 0.34) |
| Black | 11/48 (23) | 6/20 (30) | 25.8 | 36.8 | 0.63 (0.23, 1.72) |
| Asian | 14/93 (15) | 18/47 (38) | NE | 18.5 | 0.33 (0.16, 0.67) |
| Others | 38/141 (27) | 27/58 (47) | 30 | 18.4 | 0.40 (0.24, 0.65) |
| Age | |||||
| 19/106 (18) | 25/43 (58) | NE | 7.3 | 0.14 (0.08, 0.27) | |
| 65- | 75/307 (24) | 88/169 (52) | NE | 14.6 | 0.25 (0.18, 0.34) |
| ≥75 | 90/393 (23) | 81/189 (43) | 40.5 | 18.5 | 0.42 (0.31, 0.56) |
| Region | |||||
| North America | 70/285 (25) | 67/134 (50) | 40.5 | 15.7 | 0.30 (0.21, 0.42) |
| Europe | 93/395 (24) | 101/204 (50) | NE | 14.8 | 0.29 (0.22, 0.39) |
| Rest of the world | 21/126 (17) | 26/63 (41) | NE | 18.5 | 0.30 (0.17, 0.54) |
HR=hazard ratio; CI=confidence interval; NE=non-estimable
1HR is estimated from an unstratified Cox proportional hazards model
Adapted from FDA review
What are the possible side effects?
ERLEADA may cause serious side effects including falls and bone fractures, and seizures.
The most common side effects of ERLEADA include feeling tired, elevated blood pressure, rash, and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The tables below show the side effects and laboratory abnormalities noted in 1201 patients who received at least one dose of study treatment and were included in the safety assessment (safety population).
Table 4. Adverse Reactions Occurring in ≥10% of Patients in ERLEADA Clinical Trial
| ERLEADA N=803 | Placebo N=398 | |||||||
|---|---|---|---|---|---|---|---|---|
| System/Organ Class Adverse reaction | All Grades % | Grade 3-4 % | All Grades % | Grade 3-4 % | ||||
| General disorders and administration site conditions | ||||||||
| Fatigue1,4 | 39 | 1 | 28 | 0.3 | ||||
| Musculoskeletal and connective tissue disorders | ||||||||
| Arthralgia4 | 16 | 0 | 8 | 0 | ||||
| Skin and subcutaneous tissue disorders | ||||||||
| Rash2 | 24 | 5 | 6 | 0.3 | ||||
| Metabolism and nutrition disorders | ||||||||
| Decreased appetite5 | 12 | 0.1 | 9 | 0 | ||||
| Peripheral edema6 | 11 | 0 | 9 | 0 | ||||
| Injury, poisoning and procedural complications | ||||||||
| Fall4 | 16 | 2 | 9 | 0.8 | ||||
| Fracture3 | 12 | 3 | 7 | 0.8 | ||||
| Investigations | ||||||||
| Weight decreased4 | 16 | 1 | 6 | 0.3 | ||||
| Vascular disorders | ||||||||
| Hypertension | 25 | 14 | 20 | 12 | ||||
| Hot flush | 14 | 0 | 9 | 0 | ||||
| Gastrointestinal disorders | ||||||||
| Diarrhea | 20 | 1 | 15 | 0.5 | ||||
| Nausea | 18 | 0 | 16 | 0 | ||||
1 Includes fatigue and asthenia
2 Includes rash, rash maculo-papular, rash generalized, urticaria, rash pruritic, rash macular, conjunctivitis, erythema multiforme, rash papular, skin exfoliation, genital rash, rash erythematous, stomatitis, drug eruption, mouth ulceration, rash pustular, blister, papule, pemphigoid, skin erosion, and rash vesicular
3 Includes rib fracture, lumbar vertebral fracture, spinal compression fracture, spinal fracture, foot fracture, hip fracture, humerus fracture, thoracic vertebral fracture, upper limb fracture, fractured sacrum, hand fracture, pubis fracture, acetabulum fracture, ankle fracture, compression fracture, costal cartilage fracture, facial bones fracture, lower limb fracture, osteoporotic fracture, wrist fracture, avulsion fracture, fibula fracture, fractured coccyx, pelvic fracture, radius fracture, sternal fracture, stress fracture, traumatic fracture, cervical vertebral fracture, femoral neck fracture, and tibia fracture
4 Grade 4 definitions do not exist for these reactions
5 Includes appetite disorder, decreased appetite, early satiety, and hypophagia
6 Includes peripheral edema, generalized edema, edema, edema genital, penile edema, peripheral swelling, scrotal edema, lymphedema, swelling, and localized edema
ERLEADA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: All the patients were men since ERLEADA is for the treatment of prostate cancer.
- Race: The occurrence of side effects was similar in White, Black or African American or Asian patients.
- Age: The occurrence of more severe side effects (grade 3-4) in patients treated with ERLADA was higher as age increases.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Grade 3-4 adverse reactions occurred in 46% (323/697) of patients 65 years or older and in 51% (197/391) of patients 75 years or older treated with ERLEADA compared to 35% (124/355) of patients 65 years or older and 37% (70/187) of patients 75 years or older treated with placebo.
The table below shows frequent adverse reactions (all grades) by race and age subgroups noted in the clinical trial.
Table 5: Frequent Adverse Reactions by Race and Age Subgroups
| ERLEADA n/N (%) | Placebo n/N (%) | |
|---|---|---|
| Fatigue1 | ||
| Race | ||
| Asian | 19/92 (21) | 2/47 (4) |
| Black/African American | 11/48 (23) | 6/20 (30) |
| White | 221/522 (42) | 77/273 (28) |
| Other/Not Reported | 62/141 (44) | 25/58 (43) |
| Age | ||
| 65> | 42/106 (40) | 12/43 (28) |
| 65-75> | 115/306 (38) | 46/168 (27) |
| 75 and older | 156/391 (40) | 52/187 (28) |
| Arthralgia | ||
| Race | ||
| Asian | 5/92 (5) | 1/47 (2) |
| Black/African American | 10/48 (21) | 1/20 (5) |
| White | 85/522 (16) | 26/273 (9) |
| Other/Not Reported | 29/141 (21) | 5/58 (9) |
| Age | ||
| 65> | 16/106 (15) | 3/43 (7) |
| 65-75> | 50/306 (16) | 13/168 (8) |
| 75 and older | 63/391 (16) | 17/187 (9) |
| Rash2 | ||
| Race | ||
| Asian | 29/92 (31) | 1/47 (2) |
| Black/African American | 7/48 (15) | 1/20 (5) |
| White | 114/522 (22) | 17/273 (4) |
| Other/Not Reported | 28/141 (20) | 2/58 (3) |
| Age | ||
| 65> | 19/106 (18) | 4/43 (9) |
| 65-75> | 55/306 (18) | 6/168 (6) |
| 75 and older | 104/391 (27) | 11/187 (6) |
| Fall | ||
| Race | ||
| Asian | 2/92 (2) | 3/47 (6) |
| Black/African American | 7/48 (15) | 2/20 (10) |
| White | 92/522 (18) | 26/273 (10) |
| Other/Not Reported | 28/141 (20) | 5/58 (9) |
| Age | ||
| 65> | 14/106 (13) | 1/43 (2) |
| 65-75> | 38/306 (12) | 10/168 (6) |
| 75 and older | 77/391 (20) | 25/187 (13) |
| Fracture3 | ||
| Race | ||
| Asian | 8/92 (9) | 5/47 (11) |
| Black/African American | 2/48 (4) | 0/20 (0) |
| White | 52/522 (10) | 17/273 (6) |
| Other/Not Reported | 20/141 (14) | 3/58 (5) |
| Age | ||
| 65> | 9/106 (8) | 2/43 (5) |
| 65-75> | 25/306 (8) | 5/168 (3) |
| 75 and older | 48/391 (12) | 48/187 (26) |
1Includes fatigue, asthenia
2Includes rash, rash maculo-papular, rash generalized, urticaria, rash pruritic, rash macular, conjunctivitis, erythema multiforme, rash papular, skin exfoliation, genital rash, rash erythematous, stomatitis, drug eruption, mouth ulceration, rash pustular, blister, papule, pemphigoid, skin erosion, and rash vesicular.
3Includes rib fracture, lumbar vertebral fracture, spinal compression fracture, spinal fracture, foot fracture, hip fracture, humerus fracture, thoracic vertebral fracture, upper limb fracture, fractured sacrum, hand fracture, pubis fracture, acetabulum fracture, ankle fracture, compression fracture, costal cartilage fracture, facial bones fracture, lower limb fracture, osteoporotic fracture, wrist fracture, avulsion fracture, fibula fracture, fractured coccyx, pelvic fracture, radius fracture, sternal fracture, stress fracture, traumatic fracture, cervical vertebral fracture, femoral neck fracture, tibia fracture.
Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved ERLEADA based on evidence from a clinical trial (NCT01946204) of 1207 patients with prostate cancer that had not spread outside the prostate and that had not responded to castration. The trial was conducted at 332 sites in 26 countries (United States, Canada, several European countries, Israel, Japan, South Korea and Taiwan).
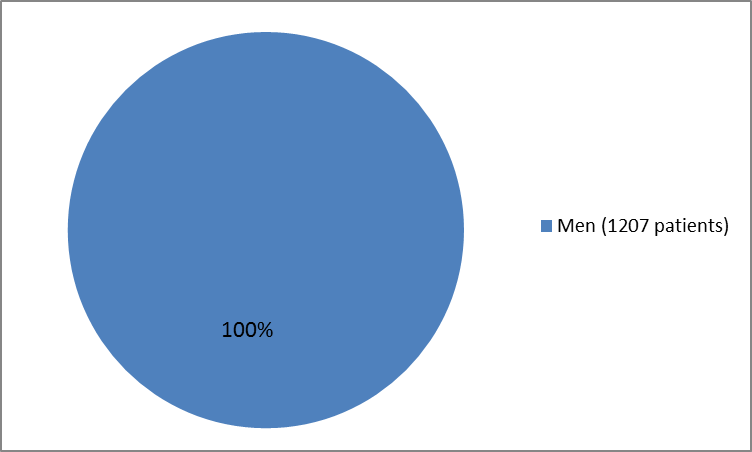
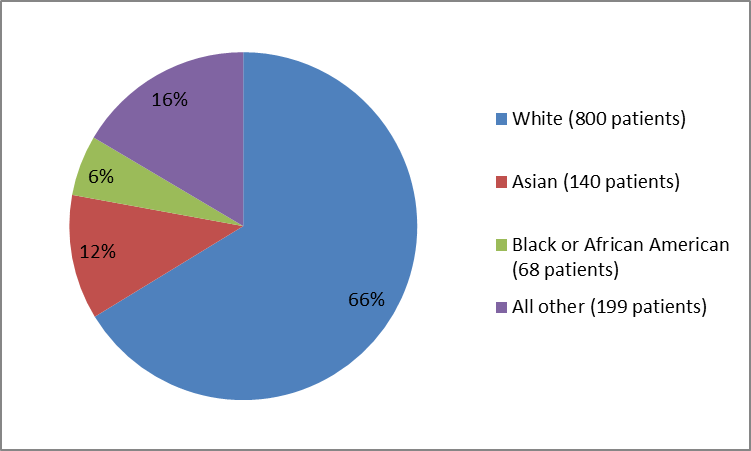
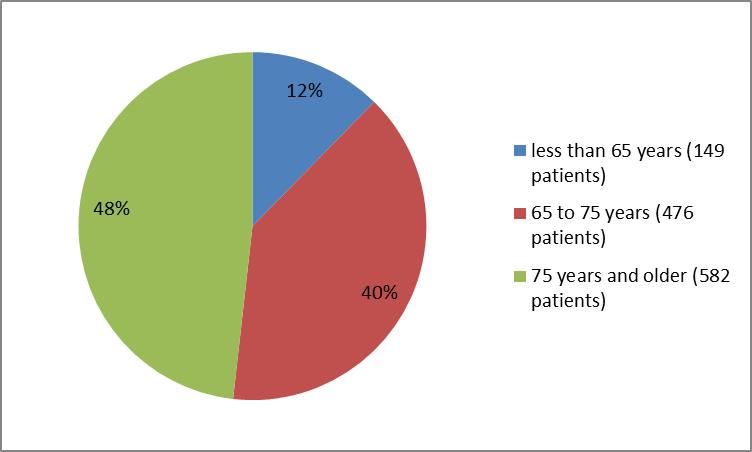
Figures 1, 2, and 3 summarize how many were in the clinical trial by sex, race and age.
Figure 1. Baseline Demographics by Sex
FDA review
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics by Race
| Number of Patients | Percent | |
|---|---|---|
| Race | ||
| White | 800 | 66 |
| Asian | 140 | 12 |
| Black or African American | 68 | 6 |
| American Indian or Alaska Native | 4 | less than 1 |
| Other | 2 | less than 1 |
| Multiple | 1 | less than 1 |
| Not Reported* | 192 | 16 |
* Data on race and/or ethnicity were not collected in some countries because of local regulations
FDA Review
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the Trials?
The table below shows the trial demographics.
Table 8. Trial Demographics
| ERLEADA N=806 (%) | Placebo N=401 (%) | |
|---|---|---|
| Sex | ||
| Men | 806 (100) | 401 (100) |
| Race | ||
| White | 524 (65) | 276 (69) |
| Asian | 93 (12) | 47 (12) |
| Black or African American | 48 (6) | 20 (5) |
| American Indian or Alaska Native | 4 (0.5) | 0 (0) |
| Multiple | 1 (0.1) | 0 (0) |
| Other | 1 (0.1) | 1 (0.2) |
| Not Reported* | 135 (17) | 57 (14) |
| Age (years) | ||
| Median (Min, Max) | 74 (48 - 94) | 74 (52 - 97) |
| 106 (13) | 43 (11) | |
| 65- | 307 (38) | 169 (42) |
| ≥75 | 393 (48) | 189 (47) |
| Ethnicity | ||
| Hispanic or Latino | 11 (1.4) | 5 (1.2) |
| Not Hispanic or Latino | 659 (82) | 338 (84) |
| Not Reported* | 136 (17) | 58 (14) |
| Geographic Region | ||
| USA | 224 (28) | 113 (28) |
| Rest of the World | ||
| Europe | 395 (49) | 204 (51) |
| Asia | 83 (10) | 43 (11) |
| Canada | 61 (8) | 21 (5) |
| Australia / New Zealand | 36 (4.5) | 13 (3.2) |
| Israel | 7 (0.9) | 7 (1.7) |
*Data on race and/or ethnicity were not collected in some countries because of local regulations
FDA Review
How were the trials designed?
There was one trial that assessed efficacy and side effects of ERLEADA. Al patients in the trial had a prostate cancer that had not spread outside the prostate and that had not responded to castration. Patients were randomly assigned to receive ERLEADA or placebo. Neither the patient nor the healthcare provider knew which treatment was being given until after the trial was completed. All patients also received either hormone therapy or surgery to lower the amount of testosterone in their body.
The efficacy of ERLEADA was assessed by measuring the length of time that tumor did not spread to other parts of the body or that death occurred after starting treatment. That is called metastasis-free survival (MFS).
How were the trials designed?
Patients with non-metastatic castration resistant prostate cancer (NM-CRPC) were randomized 2:1 to receive either ERLEADA orally at a dose of 240 mg once daily or placebo in a multicenter, double-blind, clinical trial (NCT01946204). All patients received concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. The primary efficacy outcome was metastasis-free survival (MFS), defined as the time from randomization to the time of first evidence of blinded independent central review (BICR)-confirmed distant metastasis, defined as new bone or soft tissue lesions or enlarged lymph nodes above the iliac bifurcation, or death due to any cause, whichever occurred first. Additional efficacy endpoints were time to metastasis (TTM), progression-free survival (PFS) time to symptomatic progression, and overall survival (OS).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION