Drug Trial Snapshot: QINLOCK
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the QINLOCK Prescribing Information for complete information.
QINLOCK (ripretinib)
(kin-lok)
Deciphera Pharmaceuticals, Inc.
Approval date: May 15, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
QINLOCK is drug used to treat adult patients with gastrointestinal stromal tumor (GIST) whose disease:
- cannot be surgically removed or,
- has spread throughout the body (metastatic GIST), and
- has been treated with at least three prior treatments.
GIST is type of stomach, bowel, or esophagus tumor.
How is this drug used?
QINLOCK is a tablet taken once daily.
What are the benefits of this drug?
The benefit of QINLOCK was evaluated by measuring the length of time tumors did not grow after treatment (progression-free survival or PFS). The progression-free survival for patients taking QINLOCK was about 6 months compared to 1 month for patients taking a placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results of the trial based on progression-free survival (PFS) based on disease assessment using modified RECIST 1.1 guidelines. Additional efficacy outcome measures were overall response rate (ORR) and overall survival (OS).
Table 1. Efficacy Results
|
QINLOCK |
Placebo |
|
|---|---|---|
|
Progression-Free Survivala |
||
|
Number of events (%) |
51 (60) |
37 (84) |
|
Progressive disease |
46 (54) |
32 (73) |
|
Deaths |
5 (6) |
5 (11) |
|
Median PFS (months) (95% CI) |
6.3 (4.6, 6.9) |
1.0 (0.9, 1.7) |
|
Hazard ratio (95% CI)c |
0.15 (0.09, 0.25) |
|
|
p-valueb |
< 0.0001 |
|
|
Overall Response Ratea |
||
|
Overall Response Rate (%) |
9 |
0 |
|
(95% CI) |
(4.2, 18) |
(0, 8) |
|
p-valued |
0.0504 |
|
|
Overall Survivale |
||
|
Number of deaths (%) |
26 (31) |
26 (59) |
|
Median OS (months) (95% CI) |
15 (12, 15) |
7 (4.1, 12) |
|
Hazard ratio (95% CI)c |
0.36 (0.21, 0.62) |
|
BICR=Blinded Independent Central Review; CI=Confidence Interval
a. Assessed per BICR.
b. p-value is based on 2-sided stratified log-rank test.
c. Hazard ratio is based on Cox proportional regression model. This model includes treatment and randomization stratification factors as fixed factors.
d. Based on Fisher’s exact test. The p-value is not statistically significant.
e. Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints of ORR and OS.
QINLOCK Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: QINLOCK worked similarly between men and women.
- Race: The majority of patients in the clinical trial were White. Differences among races could not be determined because of the small number of patients of other races.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to QINLOCK between patients below and above 65 years of age could not be determined because of the small number of patients older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
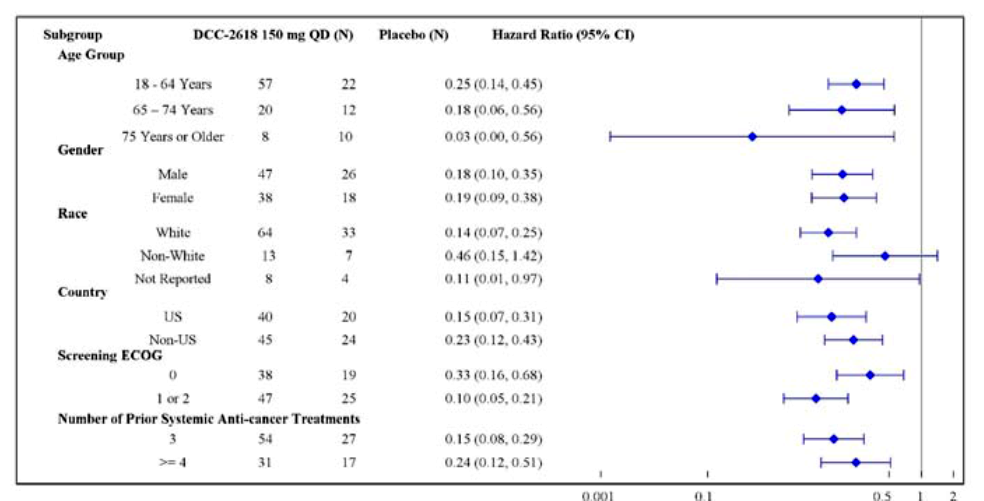
The table below summarizes efficacy results based PFS by sex, race, and age. Because of the small sample sizes, the exploratory analyses should be interpreted with caution.
Figure 5. Efficacy Analyses by Subgroups
CI = confidence interval; DCC‐2618 =QINLOCK; ECOG = Eastern Cooperative Oncology Group; ITT = intention‐to‐treat.
FDA Review
What are the possible side effects?
QINLOCK can cause serious side effects including skin reaction in the palms and soles (called palmar-plantar erythrodysesthesia syndrome), new skin cancers, high blood pressure, weakening of the heart function, delayed wound healing, and harm to a newborn baby.
The most common side effects of QINLOCK are hair loss, tiredness, nausea, abdominal pain, constipation, muscle pain, diarrhea, decreased appetite, palmar-plantar erythrodysesthesia, and vomiting.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial.
Table 2. Adverse Reactions (≥ 10%) in Patients Who Received QINLOCK
|
Adverse Reaction |
QINLOCK |
Placebo |
||
|---|---|---|---|---|
|
Grades 1-4 |
Grade 3-4 |
Grades 1-4 |
Grade 3-4 |
|
|
Skin and subcutaneous tissue |
||||
|
Alopecia |
52 |
0 |
4.7 |
0 |
|
Palmar-plantar erythrodysesthesia |
21 |
0 |
0 |
0 |
|
Dry skin |
13 |
0 |
7 |
0 |
|
Pruritus |
11 |
0 |
4.7 |
0 |
|
General |
||||
|
Fatigue |
42 |
3.5 |
23 |
2.3 |
|
Peripheral edema |
17 |
1.2 |
7 |
0 |
|
Asthenia |
13 |
1.2 |
14 |
4.7 |
|
Gastrointestinal |
||||
|
Nausea |
39 |
3.5 |
12 |
0 |
|
Abdominal pain |
36 |
7 |
30 |
4.7 |
|
Constipation |
34 |
1.2 |
19 |
0 |
|
Diarrhea |
28 |
1.2 |
14 |
2.3 |
|
Vomiting |
21 |
3.5 |
7 |
0 |
|
Stomatitis |
11 |
0 |
0 |
0 |
|
Musculoskeletal and connective tissue |
||||
|
Myalgia |
32 |
1.2 |
12 |
0 |
|
Arthralgia |
18 |
0 |
4.7 |
0 |
|
Muscle spasms |
15 |
0 |
4.7 |
0 |
|
Metabolism and nutrition |
||||
|
Decreased appetite |
27 |
1.2 |
21 |
2.3 |
|
Investigations |
||||
|
Decreased weight |
19 |
0 |
12 |
0 |
|
Nervous system |
||||
|
Headache |
19 |
0 |
4.7 |
0 |
|
Vascular |
||||
|
Hypertension |
14 |
7 |
4.7 |
0 |
|
Respiratory, thoracic and mediastinal |
||||
|
Dyspnea |
13 |
0 |
0 |
0 |
QINLOCK Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients in the clinical trial were White. Differences in side effects among races could not be determined because of the small number of patients of other races.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined because of the small number of patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize adverse events (AEs) occurring in the clinical trial by sex, and age subgroups. Race analysis was not conducted because of predominance of Whites.
Table 3. Occurrence of AEs by Sex
|
QINLOCK (N=85) |
||
|---|---|---|
|
Women |
Men |
|
|
Any TEAE |
38 (100) |
46 (98) |
|
Grade 3-4 TEAE |
21 (55) |
21 (45) |
|
Serious AE |
11 (29) |
15 (32) |
Table 4. Occurrence of AEs by Age
|
QINLOCK (N=85) |
||
|---|---|---|
|
<65 years |
≥65 years |
|
|
Any TEAE |
57 (100) |
27 (96) |
|
Grade 3-4 TEAE |
28 (49) |
15 (50) |
|
Serious AE |
15(26) |
11 (39) |
TEAE=treatment-emergent AE
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved QINLOCK based primarily on evidence from one clinical trial (NCT03353753) of 129 patients with GIST. The trial was conducted at 29 sites in the United States, Australia, Belgium, Canada, France, Germany, Italy, Netherlands, Poland, Singapore, Spain, and United Kingdom.
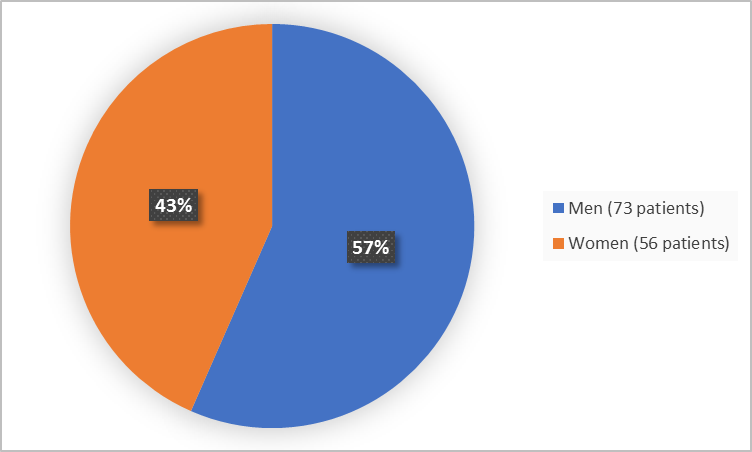
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
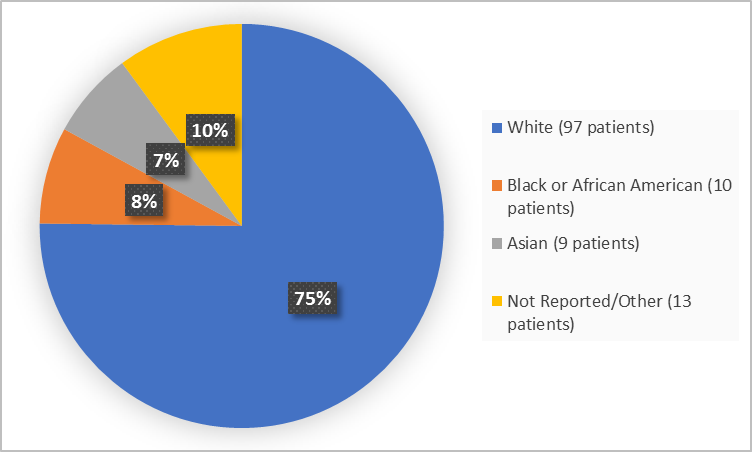
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
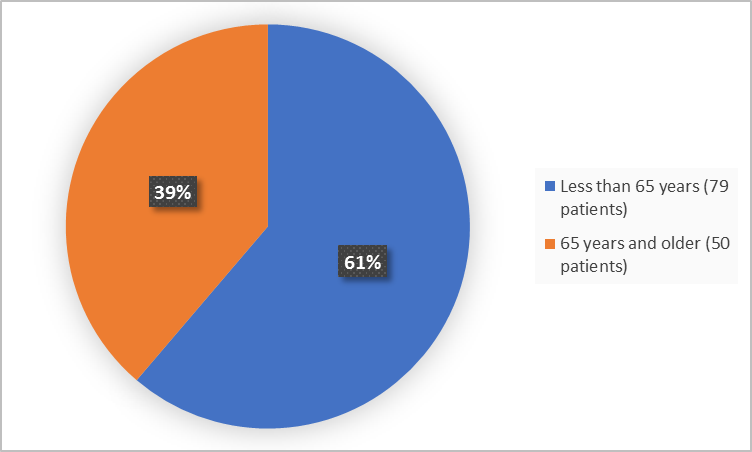
Figure 3 summarizes how many patients of certain age were in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
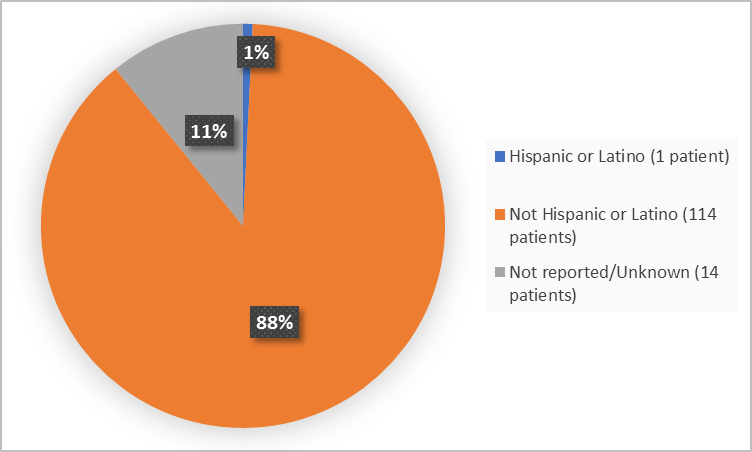
Figure 4. Baseline Demographics by Ethnicity
FDA Review
Who participated in the trials?
The table below summarizes demographics of trial population.
Table 5. Demographics of Patients in the Clinical Trial
|
|
QINLOCK |
Placebo |
Total |
|---|---|---|---|
|
Sex, n(%) |
|||
|
Women |
38 (44.7) |
18 (40.9) |
56 (43.4) |
|
Men |
47 (55.3) |
26 (59.1) |
73 (56.6) |
|
Race, n(%) |
|||
|
Asian |
4 (4.7) |
5 (11.4) |
9 (7.0) |
|
Black or African American |
8 (9.4) |
2 (4.5) |
10 (7.8) |
|
White |
64 (75.3) |
33 (75.0) |
97 (75.2) |
|
Not Reported |
8 (9.4) |
4 (9.1) |
12 (9.3) |
|
Other |
1 (1.2) |
0 |
1 (0.8) |
|
Age (years) |
|||
|
Mean (SD) |
59.1 (10.84) |
62.0 (13.50) |
60.1 (11.84) |
|
Median |
59.0 |
64.5 |
60.0 |
|
Min, Max |
29, 82 |
33, 83 |
29, 83 |
|
Age Category , n(%) |
|||
|
18 ‐ 64 Years |
57 (67.1) |
22 (50.0) |
79 (61.2) |
|
65 ‐ 74 Years |
20 (23.5) |
12 (27.3) |
32 (24.8) |
|
75 Years or Older |
8 (9.4) |
10 (22.7) |
18 (14.0) |
|
Ethnicity, n(%) |
|||
|
Hispanic or Latino |
1 (1.2) |
0 |
1 (0.8) |
|
Not Hispanic or Latino |
76 (89.4) |
38 (86.4) |
114 (88.4) |
|
Not Reported |
5 (5.9) |
5 (11.4) |
10 (7.8) |
|
Unknown |
3 (3.5) |
1 (2.3) |
4 (3.1) |
|
Region, n(%) |
|||
|
US |
40 (47.1) |
20 (45.5) |
60 (46.5) |
|
All Other |
45 (52.9) |
24 (54.5) |
69 (53.5) |
FDA Review
How were the trials designed?
There was one trial that evaluated the benefits and side effects of QINLOCK. Patients had unresectable or metastatic GIST and have received at least three previous treatments for their cancer.
Patients received either QINLOCK or placebo tablet once daily until disease progression or unacceptable side effects.
The benefit of QINLOCK was evaluated by comparing the length of time tumors did not grow after treatment (progression-free survival or PFS) between the patients who received QINLOCK and those who received placebo.
How were the trials designed?
The safety and efficacy of QINLOCK for the treatment of GIST were primarily evaluated in one trial.
This was a multicenter, randomized, double-blind, placebo-controlled trial in adult patients with unresectable or metastatic GIST who had received prior treatment with imatinib, sunitinib, and regorafenib. QINLOCK 150 mg or placebo were administered orally once daily until disease progression or unacceptable toxicity. Tumor response assessments were performed every 28 days through for the first four months and then every 56 days thereafter.
The primary endpoint was PFS based on disease assessment by BICR using modified RECIST 1.1 criteria. Additional efficacy outcome measures included objective response rate (ORR) by BICR and overall survival (OS).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.