Drug Trial Snapshot: REVCOVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the REVCOVI Package Insert for complete information.
REVCOVI (elapegademase-lvlr)
Rev-KOH-vee
Lediant Biosciences Inc.
Approval date: October 5, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
REVCOVI is a drug used to treat adenosine deaminase severe combined immune deficiency (ADA-SCID) in pediatric and adult patients. REVCOVI replaces the missing ADA enzyme in patients with ADA-SCID.
ADA-SCID is a rare, inherited, genetic disorder caused by a deficiency in the adenosine deaminase (ADA) enzyme. Patients affected by ADA-SCID have compromised immune systems that leave them unprotected from infection. The condition can be life threatening if left untreated.
How is this drug used?
REVCOVI is injected into the muscle (an intramuscular injection) weekly.
The dose depends on the patient’s weight.
What are the benefits of this drug?
REVCOVI increases ADA enzyme activity.
What are the benefits of this drug (results of trials used to assess efficacy)?
Clinical efficacy results for the clinical trials are summarized below using the following endpoints:
- Trough dAXP Level (metabolic detoxification was defined as a trough erythrocyte dAXP concentration equal to or below 0.02 mmol/L)
- Trough plasma ADA activity (adequate trough plasma ADA activity is defined as trough plasma ADA activity equal to or above 15 mmol/hr/L)
- Immune status (lymphocyte and B-, T-, and NK-lymphocyte subset counts as well as quantitative immunoglobulin [Ig] concentration [IgG, IgA, IgM])
REVCOVI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The number of patients in the trial was small; therefore, differences in how well the drug worked among sex, race, and age subgroups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The number of patients in the trials was too low to allow for meaningful demographic subgroup analysis.
What are the possible side effects?
REVCOVI may cause serious side effects including injection site bleeding in patients with low platelet counts. The most common side effects are cough and vomiting.
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions in patients with ADA-SCID are summarized below.
REVCOVI Prescribing Information
Were there any differences in side effects among sex, race and age?
The number of patients in the trial was small, and the number of patients who experienced side effects in each subgroup was limited; therefore, differences in side effect among sex, race and age subgroups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The occurrence of side effects per demographic subgroup was too low to allow for meaningful analysis.
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved REVCOVI based on evidence from two clinical trials (Trial 1/ NCT01420627 and Trial 2/STM-279-301) of ten patients with adenosine deaminase severe combined immune deficiency (ADA-SCID). Trial 1 was conducted in the United States and Trial 2 in Japan.
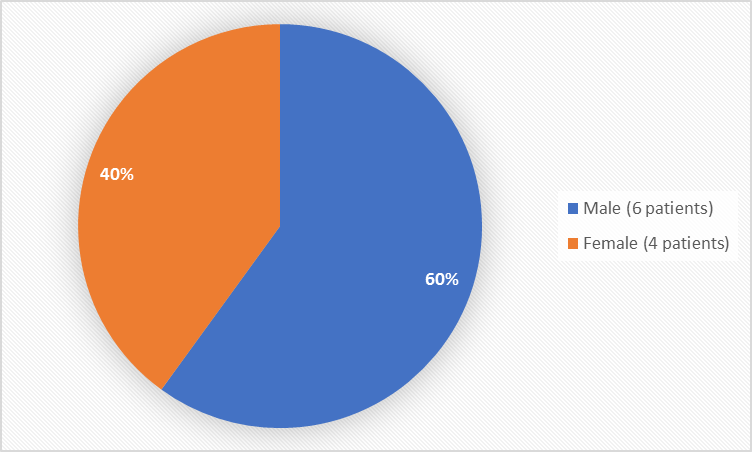
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
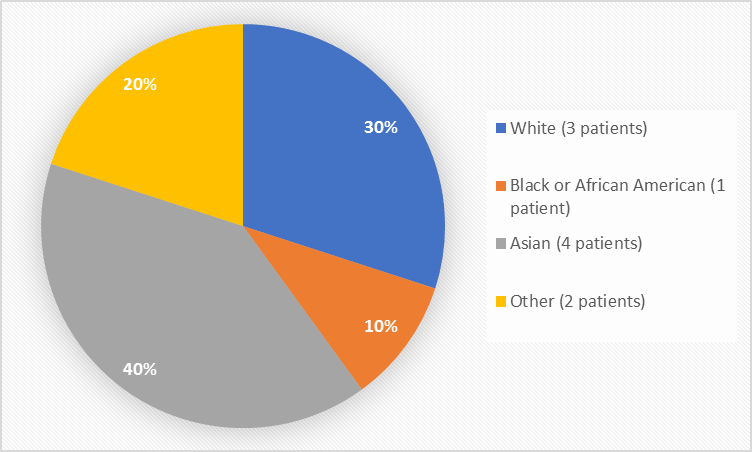
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Efficacy Trials by Race
|
Race |
Number of Patients |
Percentage of Patients |
|---|---|---|
|
White |
3 |
30% |
|
Black or African American |
1 |
10% |
|
Asian |
4 |
40% |
|
Other |
2 |
20% |
FDA Review
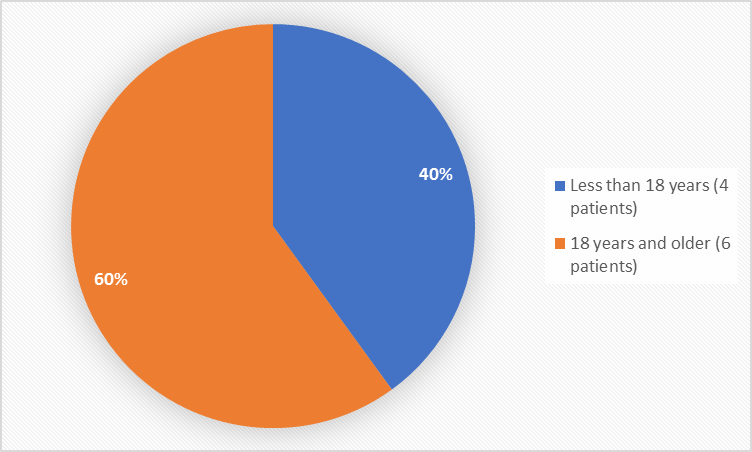
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 2. Baseline Demographics of Patients in the Clinical Trials
|
Demographic Parameters |
Trial 1 |
Trial 2 |
TOTAL |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Male |
4 (67) |
2 (50) |
6 (60) |
|
Female |
2 (33) |
2 (50) |
4 (40) |
|
Race, n (%) |
|||
|
White |
3 (50) |
0 (0) |
3 (30) |
|
Black or African American |
1 (17) |
0 (0) |
1 (10) |
|
Asian |
0 (0) |
4 (100) |
4 (40) |
|
Other |
2 (33) |
0(0) |
2 (20) |
|
Age Group, n (%) |
|||
|
Less than 18 years |
1 (17) |
3 (75) |
4 (40) |
|
18 years and older |
5 (83) |
1 (25) |
6 (60) |
| Ethnicity, n (%) | |||
|
Hispanic |
3 (50) |
0 (0) |
3 (30) |
|
Non-Hispanic |
3 (50) |
4 (100) |
7 (70) |
| Region, n (%) | |||
|
Japan |
0 (0) |
4 (100) |
4 (40) |
|
United States |
6 (100) |
0 (0) |
6 (60) |
FDA Review
How were the trials designed?
The benefit and side effects of REVCOVI were evaluated in two clinical trials in adult and pediatric patients with adenosine deaminase severe combined immune deficiency (ADA-SCID).
Trial 1 enrolled patients who were receiving adagen (enzyme replacement therapy developed from horse enzymes) for ADA-SCID. All patients received a once weekly IM dose of adagen for at least 3 weeks. This was followed by weekly IM doses of REVCOVI for 21 weeks. The benefit of REVCOVI was assessed based on activity of the ADA enzyme activity for Weeks 15, 17, 19 and 21.
Trial 2 enrolled adult and pediatric patients with ADA-SCID. All patients received weekly IM doses of REVCOVI for 21 weeks. The benefit of REVCOVI was assessed based on activity of the ADA enzyme activity.
How were the trials designed?
The efficacy and safety of REVCOVI were established in two clinical trials. The trials evaluated REVCOVI for the treatment of ADA-SCID in pediatric and adult patients.
Trial 1 is an ongoing open-label, single-arm, one-way crossover trial in patients who are receiving adagen. The trial consists of three phases: the adagen Lead-in Phase (minimum of 3 weeks), the REVCOVI Treatment Phase (weeks 1 through 21) and is followed by the REVCOVI Maintenance Phase. Patients entered the trial in the adagen lead-in phase and received a once weekly IM dose of adagen weekly. REVCOVI doses ranged from 0.188 mg/kg to 0.292 mg/kg. The primary efficacy endpoint was the trough dAXP Level (metabolic detoxification was defined as a trough erythrocyte dAXP concentration equal to or below 0.02 mmol/L).
Trial 2 is an ongoing single-arm clinical trial that evaluated safety, efficacy, and PK in patients with ADA-SCID. The trial included two phases: 1) Evaluation, consisting of a Dose Adjustment Period (5 weeks) and a Dose Maintenance Period (16 weeks); and 2) Continuous Administration (Extension) Phase, to be continued until the end of the trial. REVCOVI was administered as a once weekly IM injection at a starting dose and dose titration based on their dosage of adagen converted to an equivalent dose of REVCOVI. Patients who had not been treated with adagen within 4 weeks before informed consent, were administered once weekly IM injection with the first dose of REVCOVI at 0.1 mg/kg body weight, the second and third doses at 0.133 mg/kg body weight. This trial did not identify a specific hypothesis to be tested using a pre-specified primary endpoint.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
REVCOVI PRESCRIBING INFORMATION