Drug Trial Snapshot: TAUVID
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TAUVID Package Insert for complete information.
TAUVID (flortaucipir F 18)
(TAAOW-vihd)
Eli Lilly and Company
Approval date: May 28, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TAUVID is a drug for the visual detection of aggregated neurofibrillary tangles or NFTs in the brain of adult patients with suspected Alzheimer’s disease (AD).
NFTs are deposits of tau protein that are present in the brains of patients with AD. Alzheimer’s disease is a common degenerative disease of the brain that starts with mild thinking, judging and memory problems and progresses to dementia and death.
How is this drug used?
TAUVID is injected into a vein by a healthcare provider in preparation for an imaging test (called positron emission tomography scan or PET scan) to detect the NFTs.
What are the benefits of this drug?
TAUVID detects NFTs on PET scan images of the brain.
What are the benefits of this drug (results of trials used to assess efficacy)?
In Trial 1, the reading of TAUVID imaging of terminally ill patients was compared to tau pathology based on scoring provided by independent pathologists, who evaluated the density and distribution of NFTs in the post-mortem brain. Image reader performance for distinguishing B3 (positive) from B0-B2 (negative) tau pathology is shown in table below.
Table 1. TAUVID Scan Reader Performance for B3 Tau Pathology in Trial 1
|
Reader |
True Positive |
True Negative |
False Positive |
False Negative |
Sensitivity % (95% CIa) |
Specificity % (95% CI) |
|---|---|---|---|---|---|---|
|
1 |
38 |
17 |
8 |
1 |
97 (87, 100) |
68 (48, 83) |
|
2 |
36 |
23 |
2 |
3 |
92 (80, 97) |
92 (75, 98) |
|
3 |
36 |
22 |
3 |
3 |
92 (80, 97) |
88 (70, 96) |
|
4 |
36 |
19 |
6 |
3 |
92 (80, 97) |
76 (57, 89) |
|
5 |
39 |
13 |
12 |
0 |
100 (91, 100) |
52 (34, 70) |
a CI = confidence interval.
Trial 2 included the same images from terminally ill patients as in Trial 1 (plus 18 additional terminally ill patients) and 159 patients with cognitive impairment being evaluated for AD (the indicated population). Inter-reader agreement for five new TAUVID readers was evaluated using Fleiss’ kappa statistic (95% CI) and found to be 0.87 (0.83, 0.91) across all 241 patients. Exploratory analysis evaluated inter-reader agreement in two subgroups. In this analysis, Fleiss’ kappa (95% CI) was 0.82 (0.75, 0.88) in the terminally ill patients and 0.90 (0.85, 0.95) in the indicated population.
TAUVID Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TAUVID worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in how TAUVID worked among races could not be determined.
- Age: TAUVID worked similarly in younger and older patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex and age groups.
Table 2. Diagnostic Performance of Individual FTP PET Scan Readers for Detection of B3 NFTs in Cases by Sex-Trial 1
|
|
Sensitivity |
Specificity |
||
|---|---|---|---|---|
|
|
Men |
Women |
Men |
Women |
|
Reader 1 |
94.4 |
100.0 |
66.7 |
69.2 |
|
(74.2, 99.0) |
(84.5, 100.0) |
(39.1, 86.2) |
(42.4, 87.3) |
|
|
Reader 2 |
83.3 |
100.0 |
91.7 |
92.3 |
|
(60.8, 94.2) |
(84.5, 100.0) |
(64.6, 98.5) |
(66.7, 98.6) |
|
|
Reader 3 |
83.3 |
100.0 |
91.7 |
84.6 |
|
(60.8, 94.2) |
(84.5, 100.0) |
(64.6, 98.5) |
(57.8, 95.7) |
|
|
Reader 4 |
83.3 |
100.0 |
75.0 |
76.9 |
|
(60.8, 94.2) |
(84.5, 100.0) |
(46.8, 91.1) |
(49.7, 91.8) |
|
|
Reader 5 |
100.0 |
100.0 |
50.0 |
53.8 |
|
(82.4, 100.0) |
(84.5, 100.0) |
(25.4, 74.6) |
(29.1, 76.8) |
|
Table 3. Majority Read of Performance for B3 Tau Pathology by Age-Trial 1
|
Age |
N |
Sensitivity |
Specificity |
|---|---|---|---|
|
≤ 83 years |
32 |
94.7 |
92.3 |
|
(84.7, 100.0) |
(77.8, 100) |
||
|
> 83 years |
32 |
90.0 |
66.7 |
|
(76.9,100) |
(40, 93.3) |
Courtesy of FDA Statistical Team
What are the possible side effects?
TAUVID is a radioactive drug which may increase the risk of lifetime radiation exposure.
The most common side effects are headache, injection site pain, and increased blood pressure.
What are the possible side effects (results of trials used to assess safety)?
The most common adverse reactions reported in the TAUVID trials are shown in Table 4.
Table 4. Adverse Reactions with a Frequency ≥0.5% in Adults Who Received TAUVID
|
Adverse Reaction |
N=1921 |
|---|---|
|
Headache |
26 (1.4%) |
|
Injection site pain |
23 (1.2%) |
|
Increased blood pressure |
15 (0.8%) |
TAUVID Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and those older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Subgroup analyses of the most common adverse reactions (ARs) are presented below.
Table 5. Common Adverse Reactions by Demographic Subgroups
|
Adverse Reaction |
ARs by Sex |
ARs by Race |
ARs by Age |
|||
|---|---|---|---|---|---|---|
|
Men |
Women |
White |
All Other |
<65 years |
≥ 65 years |
|
|
Overall n, (%) |
97 (10) |
93 (10) |
170 (10) |
19 (8) |
41 (11) |
149 (10) |
|
Headache n, (%) |
15 (2) |
11 (1) |
21 (1) |
5 (2) |
13 (3) |
13 (1) |
|
Injection site pain n, (%) |
9 (1) |
14 (1) |
18 (1) |
5 (2) |
6 (2) |
17 (1) |
|
Increased blood pressure n, (%) |
7 (1) |
8 (1) |
12 (1) |
3 (1) |
4 (1) |
11 (1) |
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TAUVID based on evidence of 1921 patients from 19 trials conducted at 322 sites in the United States, Australia, Belgium, Canada, France, Japan, Netherlands and Poland.
The figures below summarize how many patients received at least one dose of TAUVID in the clinical trials. These patients provided data for evaluation of TAUVID side effects (safety population). Some of these patients provided data for the benefit of TAUVID (efficacy population) and their demographics are presented in Tables 7 and 8 under MORE INFO #5.
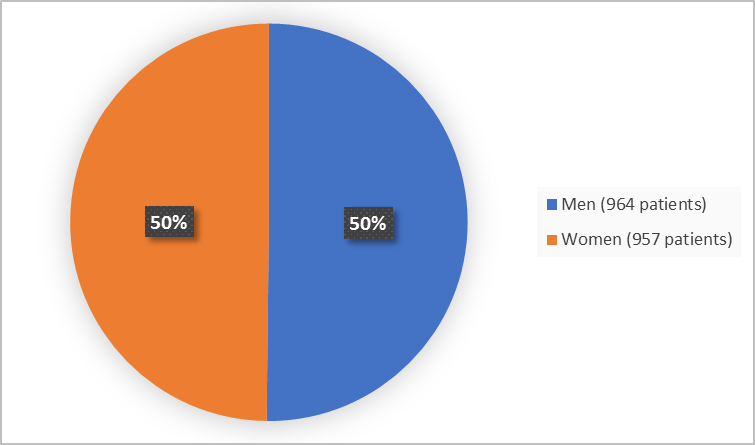
Figure 1. Demographics by Sex (safety population)
FDA Review
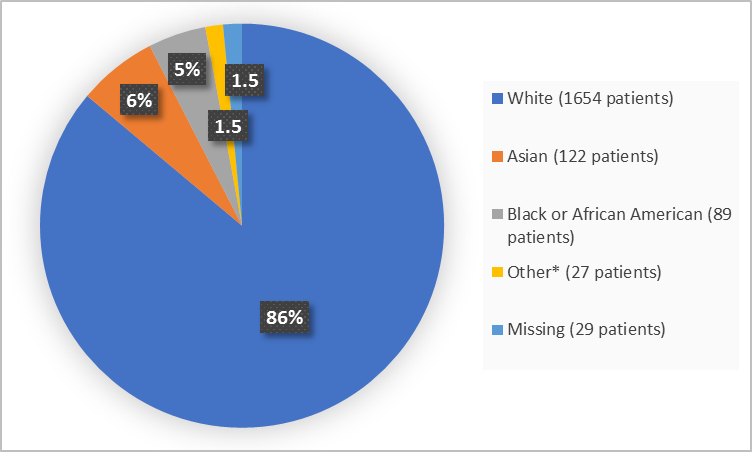
Figure 2. Demographics by Race (safety population)
*Includes American Indian or Alaska Native, Multiple, Native Hawaiian or Other Pacific Islander and Other
FDA Review
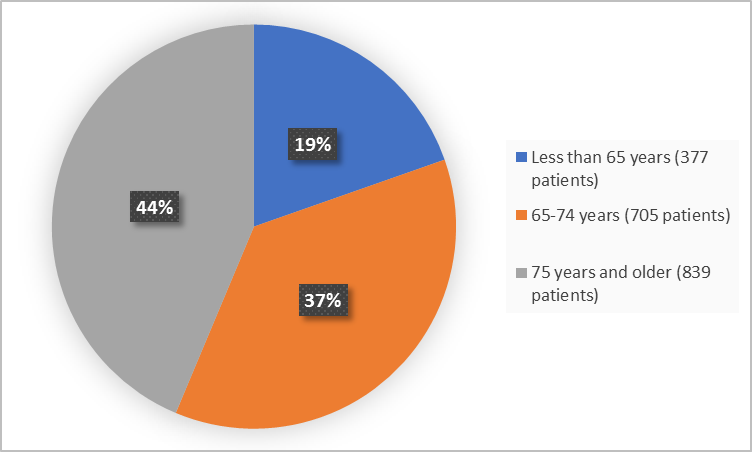
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Demographics by Age (safety population)
FDA Review
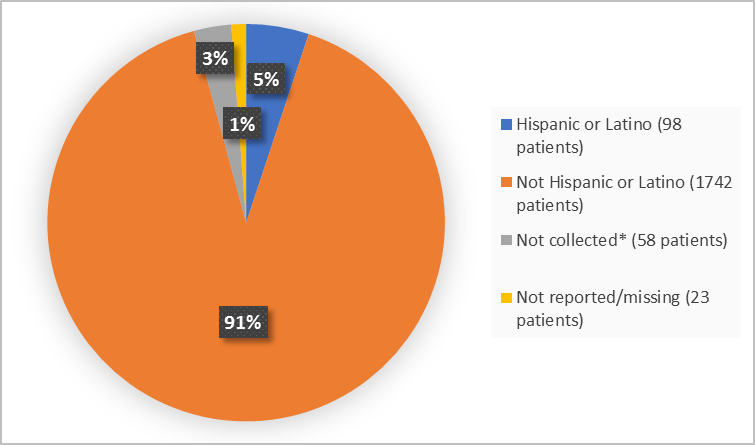
Figure 4. Demographics by Ethnicity (safety population)
* as per local regulatory authority
FDA Review
Who participated in the trials?
Table 6 summarizes demographic information for the safety population from TAUVID trials. Efficacy populations from Trials 1 and 2 are presented in Tables 7 and 8 respectively.
Table 6. Demographics (safety population)
|
Demographic Characteristic |
TOTAL |
|---|---|
|
Sex, n (%) |
|
|
Men |
964 (50.2) |
|
Women |
957 (49.8) |
|
Race, n (%) |
|
|
White |
1654 (86.1) |
|
American Indian or Alaska Native |
3 (0.2) |
|
Asian |
122 (6.4) |
|
Black or African American |
89 (4.6) |
|
Multiple |
11 (0.6) |
|
Native Hawaiian or Other Pacific Islander |
3 (0.2) |
|
Other |
10 (0.5) |
|
Missing* |
29 (1.5) |
|
Age, years |
|
|
Mean (SD) |
71.9 (10.7) |
|
Median (min, max) |
73.0 (21.0, 104.0) |
|
Age group, n (%) |
|
|
<65 years |
377(19.6) |
|
≥65 years |
1544 (80.4)) |
|
65-74 years |
705 (36.7) |
|
≥75 years |
839 (43.7) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
98 (5.1) |
|
Not Hispanic or Latino |
1742 (90.7) |
|
Not collected* |
58 (3.0) |
|
Not reported/missing |
23 (1.2) |
|
Country, n (%) |
|
|
United States |
1476 (76.8) |
|
Australia |
194 (10.1) |
|
Belgium |
14 (0.7) |
|
Canada |
23 (1.2) |
|
France |
28 (1.5) |
|
Japan |
99 (5.2) |
|
Netherlands |
21 (1.1) |
|
Poland |
66 (3.4) |
*Due to local regulatory authorities
FDA Review
Table 7. Demographics (efficacy population)-Trial 1
|
Demographic Characteristic |
TAUVID |
|---|---|
|
Sex, n (%) |
|
|
Men |
30 (46.9) |
|
Women |
34 (53.1) |
|
Race, n (%) |
|
|
White |
62 (96.9) |
|
Asian |
1 (1.6) |
|
Black or African American |
1 (1.6) |
|
Age, years |
|
|
Mean (SD) |
82.5 (9.59) |
|
Median (min, max) |
83.5 (55, 100) |
|
Age group, n (%) |
|
|
<65 years |
3 (4.7) |
|
65-74 years |
9 (14.1) |
|
≥75 years |
52 (81.3) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
3 (4.7) |
|
Not Hispanic or Latino |
61 (95.3) |
FDA Review
Table 8. Demographics (efficacy population)-Trial 2
|
Demographic Characteristic |
TAUVID |
|---|---|
|
Sex, n (%) |
|
|
Men |
86 (53.8) |
|
Women |
74 (46.3) |
|
Race, n (%) |
|
|
White |
155 (96.9) |
|
Asian |
2 (1.3) |
|
Black or African American |
3 (1.9) |
|
Age, years |
|
|
Mean (SD) |
72.9 (9.61) |
|
Median (min, max) |
73.5 (50, 97) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
9 (5.6) |
|
Not Hispanic or Latino |
151 (94.4) |
FDA Review
How were the trials designed?
The benefit of TAUVID was evaluated in two clinical trials (Trial 1/NCT02516046 and Trial 2/NCT03901092). All patients received a single intravenous dose of TAUVID before getting a PET scan to detect NFTs.
In Trial 1, PET scan images of terminally ill patients were read as positive or negative for NFTs by five readers who had no knowledge about patient’s condition and compared with brain autopsy findings of NFTs after patients’ death.
In Trial 2, all the images from Trial 1 and additional images from different patients with suspected AD (but not terminally ill) were read by five new readers. The trial analyzed how similar the readings were among different readers, and between the readings of terminally ill patients and patients with suspected AD.
How were the trials designed?
The ability of TAUVID imaging to estimate the density and distribution of NFTs was evaluated in two clinical trials.
Trial 1 enrolled 156 terminally ill patients who agreed to undergo TAUVID imaging and to participate in a postmortem brain donation program. In 64 of these patients (14 with normal, 1 with mild cognitive impairment and 49 with dementia), reader interpretation of the TAUVID scan was compared to tau pathology based on scoring provided by independent pathologists, who evaluated the density and distribution of NFTs in the post-mortem brain.
Trial 2 included the same terminally ill patients as in Trial 1 (plus 18 additional terminally ill patients) and 159 patients with cognitive impairment being evaluated for AD. Inter-reader agreement for five new TAUVID readers was evaluated using Fleiss’ kappa statistic in all 241 patients. Exploratory analysis evaluated inter-reader agreement in two subgroups: terminally ill patients and in the AD indicated population.
Safety of TAUVID was evaluated in 19 trials across drug development program.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.