Drug Trial Snapshot: TPOXX
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights subjects who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among these subjects when grouped by sex, race, and age. The efficacy of this drug was studied in animal models while the safety was studied in healthy human volunteers. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to TPOXX Prescribing Information for complete information.
TPOXX (tecovirimat)
(Tee-pahx)
SIGA Technologies
Approval date: July 13, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TPOXX is a drug for the treatment of smallpox disease.
Smallpox is a serious and highly contagious viral disease that causes fever, rash and deep skin scars and may progress to death. No cases of naturally occurring smallpox disease have happened since the late 1970s because worldwide vaccination led to the eradication of smallpox.
How is this drug used?
TPOXX is taken twice daily for 14 days. The number of capsules per day depends on patient’s weight.
What are the benefits of this drug?
More animals treated with TPOXX lived compared to the animals treated with placebo. Because smallpox disease has been eradicated from the world and it is not feasible or ethical to conduct efficacy trials in humans, the efficacy of TPOXX could not be studied in patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of TPOXX for the treatment of smallpox is based on studies in monkeys (cynomolgus macaques) and New Zealand White (NZW) rabbits. The primary efficacy endpoint was proportion of infected animals that survived to the pre-specified end-of-study.
It was not possible to conduct controlled clinical trials in humans with smallpox because this disease is eradicated.
Table 2. Survival Rates in TPOXX Treatment Studies in Cynomolgus Macaques and NZW Rabbits Exhibiting Clinical Signs of Orthopoxvirus Disease
| Treatment Initiationa | Survival Percentage | p-valueb | Survival Rate Differencec | |
|---|---|---|---|---|---|
Placebo | TPOXX | ||||
Cynomolgus Macaques | |||||
Study 1 | Day 4 | 0% (0/7) | 80% (4/5) | 0.0038 | 80% (20.8%, 99.5%) |
Study 2 | Day 4 | 0% (0/6) | 100% (6/6) | 0.0002 | 100% (47.1%, 100%) |
Study 3 | Day 4 | 0% (0/3) | 83% (5/6) | 0.0151 | 83% (7.5%, 99.6%) |
Day 5 | 83% (5/6) | 0.0151 | 83% (7.5%, 99.6%) | ||
Day 6 | 50% (3/6) | 0.1231 | 50% (-28.3%, 90.2%) | ||
NZW Rabbits | |||||
Study 4 | Day 4 | 0% (0/10) | 90% (9/10) | 90% (50.3%, 99.8%) | |
Study 5 | Day 4 | NAe | 88% (7/8) | NA | NA |
aDay post-challenge tecovirimat treatment was initiated
bp-value is from 1-sided Boschloo Test (with Berger-Boos modification of gamma = 0.000001) compared to placebo
cSurvival percentage in tecovirimat treated animals minus survival percentage in placebo treated animals
dExact 95% confidence interval based on the score statistic of difference in survival rates
eA placebo control group was not included in this study.
NA = Not Applicable
TPOXX Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The efficacy of TPOXX has been studied only in animals, so the differences among patients grouped by sex, race, and age could not be assessed.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Due to smallpox disease eradication and ethical considerations, clinical efficacy trials for TPOXX were not conducted in humans. Therefore, human data on efficacy and subgroup analyses are not available.
What are the possible side effects?
The side effects of TPOXX have been studied in healthy human volunteers.
TPOXX may cause lowering of blood sugar levels when co-administered with blood glucose-lowering drug repaglinide.
The most common side effects of TPOXX are headache, nausea, abdominal pain, and vomiting.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in at least 2% of participants in the TPOXX treatment group.
Table 3. Adverse Reactions Reported in ≥ 2% of Healthy Adults Receiving at Least One Dose of TPOXX 600 mg
Adverse Reaction | TPOXX | Placebo |
|---|---|---|
Headache | 12 | 8 |
Nausea | 5 | 4 |
Abdominal paina | 2 | 1 |
Vomiting | 2 | 0 |
aIncludes abdominal pain, abdominal pain upper, abdominal distension, abdominal discomfort, abdominal pain lower, epigastric pain
TPOXX Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women
- Race: The occurrence of side effects was similar between White and Black participants. Differences among other races could not be determined due to the small number of participants.
- Age: Most participants in the clinical trial were younger than 65 years of age, so differences in the occurrence of side effects between participants below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below shows the incidence of treatment-emergent adverse events (TEAEs) by subgroup.
Table 4. Subgroup Analysis of TEAEs
Subgroup | TPOXX (N=359) | Placebo (N=90) | ||||
|---|---|---|---|---|---|---|
n (%) | Total |
n (%) | Total | |||
Overall/All participants | 134 (37.3) | 359 | 30 (33.3) | 90 | ||
Sex | ||||||
Male | 48 (32.4) | 148 | 9 (25) | 36 | ||
Female | 89 (42.1) | 211 | 21(38.8) | 54 | ||
Race |
| |||||
White and Other | 105 (42.1) | 249 | 17 (27.4) | 62 | ||
Black or African American | 25 (24.7) | 101 | 12 (46.1) | 26 | ||
All Other | 4 (44.4) | 9 | 1 (50) | 2 | ||
Age Group | ||||||
18-65 years | 118 (36.5) | 323 | 26 (32.9) | 79 | ||
≥65 years | 16 (44.4) | 36 | 4 (36.4) | 11 | ||
Clinical trial data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved TPOXX based on efficacy results from animal studies and safety results from one clinical trial. The clinical trial enrolled 449 heathy adults and was conducted at 12 sites in the United States.
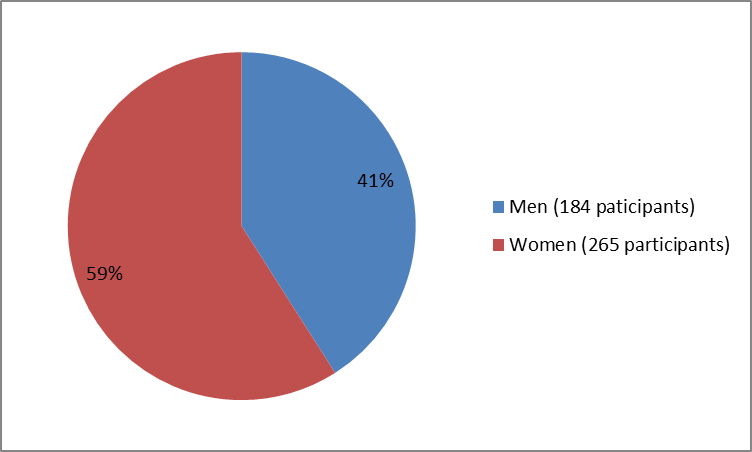
The figure below summarizes how many men and women were in the clinical trial that evaluated TPOXX safety.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 and Table 1 below summarize healthy participants by race who participated in the clinical trial that evaluated TPOXX safety.
Figure 2. Baseline Demographics by Race
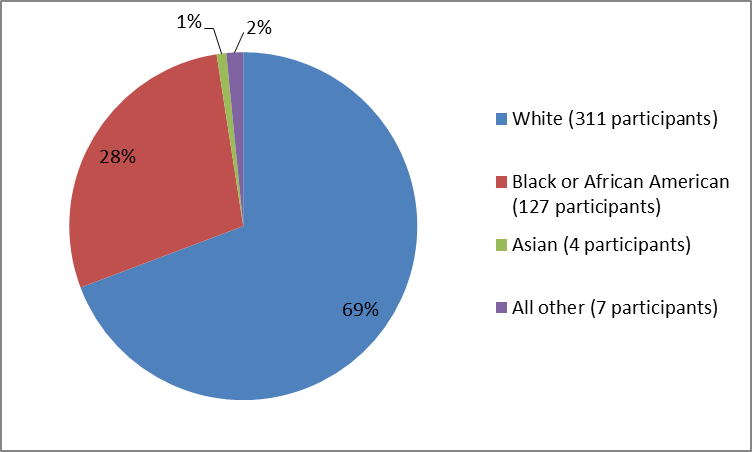
Table 1. Baseline Demographics by Race
Race | Number of Participants | Percentage |
|---|---|---|
White | 311 | 69 |
Black or African American | 127 | 28 |
Asian | 4 | 1 |
Other | 7 | 2 |
FDA Review
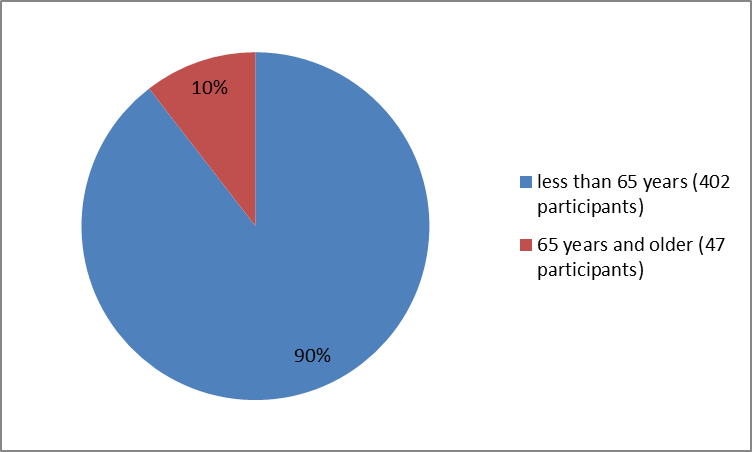
Figure 3 summarizes the percentage of healthy participants by age group who participated in the clinical trial that evaluated TPOXX safety.
Figure 3. Baseline Demographics by Age
FDA Review
The table below summarizes demographics of participants in the clinical trial.
Table 5. Baseline Demographics of Healthy Participants in the Safety Clinical Trials
Demographic Parameters | TPOXX | Placebo | Total |
|---|---|---|---|
Sex | |||
Men | 148 (41%) | 36 (40%) | 184 (41%) |
Women | 211 (59%) | 54 (60%) | 265 (59%) |
Race | |||
White | 249 (69%) | 62 (69%) | 311 (69%) |
Black or African American | 101 (28%) | 26 (29%) | 127 (28%) |
Asian | 3 (1%) | 1 (1%) | 4 (1%) |
Other1 | 6 (2%) | 1 (1%) | 7 (2%) |
Age | |||
Mean years (SD) | 40 (15.7) | 42 (15.9) | 41 (15.7) |
Median (years) | 38 | 41 | 39 |
Min, max (years) | 18, 79 | 18, 80 | 18, 80 |
Age Group | |||
| 323 (90%) | 79 (88%) | 402 (90%) |
≥ 65 years | 36 (10%) | 11 (12%) | 47 (10%) |
Ethnicity | |||
Hispanic or Latino | 43 (12%) | 5 (6%) | 48 (11%) |
Non-Hispanic or Latino | 315 (88%) | 85 (94%) | 400 (89%) |
Not disclosed | 1 ( | 0 | 1 ( |
Geographic location | |||
US | 359 (100%) | 90 (100%) | 449 (100%) |
Rest of the world | 0 | 0 | 0 |
1Includes American Indian/Alaska Native, Hawaiian or Pacific Islander and other
FDA Review
How were the trials designed?
The efficacy of TPOXX was evaluated in animal studies. The side effects of TPOXX were evaluated in one clinical trial of healthy participants. Participants received either TPOXX or placebo twice a day for 14 days. Neither the participant nor the health care providers knew which treatment was given until after the trial was completed.
How were the trials designed?
The efficacy of TPOXX for the treatment of smallpox was studied in cynomolgus macaques and NZW rabbits. The animal efficacy studies were conducted under varying conditions and compared survival rates in TPOXX and placebo treated groups.
The safety of TPOXX was studied in one randomized, double-blind, placebo-controlled clinical trial of healthy participants who were treated with TPOXX or placebo twice daily for 14 days.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.