Drug Trial Snapshot: WINLEVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the WINLEVI Package Insert for complete information.
WINLEVI (clascoterone)
(Win-levē)
Cassiopea SpA
Approval date: August 26, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
WINLEVI is used on the skin to treat acne vulgaris in patients 12 years and older.
Acne vulgaris is a skin disease characterized by oily skin, blackheads or whiteheads, pimples, and sometimes scarring.
How is this drug used?
WINLEVI is a cream. It should be applied to the affected skin area twice a day.
What are the benefits of this drug?
More patients achieved a reduction in the number of acne and clear, or almost clear, skin after 12 weeks of treatment with WINLEVI in comparison to those who were treated with vehicle cream (placebo).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the patients 12 years of age and older in Trials 1 and 2. The co-primary endpoints were the percentage of patients with Investigator’s Global Assessment (IGA) score of clear (0) or almost clear (1) with a 2-point decrease from baseline and the absolute reduction from baseline in inflammatory and non-inflammatory lesion counts at week 12.
Table 1. Clinical Efficacy of WINLEVI at Week 12
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
WINLEVI |
Vehicle |
WINLEVI |
Vehicle |
|
|
IGA Successa |
18.8% |
8.7% |
20.9% |
6.6% |
|
Difference from Vehicle (95% CI) |
10.1% (4.1%, 16.0%) |
14.3% (8.9%, 19.7%) |
||
|
Non-inflammatory Lesions |
||||
|
Mean Absolute Reduction |
20.4 |
13.0 |
19.5 |
10.8 |
|
Difference from Vehicle (95% CI) |
7.3 (3.5, 11.1) |
8.7 (4.5, 12.4) |
||
|
Mean Percent Reduction |
32.6% |
21.8% |
29.6% |
15.7% |
|
Difference from Vehicle (95% CI) |
10.8% (3.9%, 17.6%) |
13.8% (7.5%, 20.1%) |
||
|
Inflammatory Lesions |
||||
|
Mean Absolute Reduction |
19.3 |
15.4 |
20.1 |
12.6 |
|
Difference from Vehicle (95% CI) |
3.9 (1.3, 6.5) |
7.5 (5.2, 9.9) |
||
|
Mean Percent Reduction |
44.6% |
36.3% |
47.1% |
29.7% |
|
Difference from Vehicle (95% CI) |
8.3% (2.2%, 14.4%) |
17.5% (11.8%, 23.1%) |
||
a Investigator Global Assessment (IGA) success was defined as at least a 2-point reduction in IGA compared to baseline and an IGA score of 0 (clear) or 1 (almost clear).
WINLEVI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: WINLEVI worked similarly in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how WINLEVI worked among races could not be determined.
- Age: WINLEVI worked similarly in patients 12-17 years of age and 18 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figures below summarize the results for IGA success at Week 12 by subgroups in individual trials.
Figure 5. IGA Success at Week 12 by Sex, Race, and Age for Trial 1 [ITT]
Figure 6. IGA Success at Week 12 by Sex, Race, and Age for Trial 2 [ITT]
FDA Review
What are the possible side effects?
WINLEVI may cause local skin irritation, adrenal gland suppression and elevated serum potassium.
The most common side effect of WINLEVI are reddening, itching, and scaling or dryness of the skin being treated.
What are the possible side effects (results of trials used to assess safety)?
Local skin reactions observed during the12-week treatment are presented below.
Table 2. Incidence of New or Worsening Local Skin Reactions Reported by ≥ 1% of Patients Treated with WINLEVI Cream After Day 1 in 12 Week Controlled Clinical Trials
|
|
WINLEVI Cream 1% (N=674a) |
Vehicle Cream (N=656a) |
|---|---|---|
|
Edema |
24 (3.6%) |
23 (3.5%) |
|
Erythema/redness |
82 (12.2%) |
101 (15.4%) |
|
Pruritus |
52 (7.7%) |
54 (8.2%) |
|
Scaling/dryness |
71 (10.5%) |
68 (10.4%) |
|
Skin atrophy |
11 (1.6%) |
17 (2.6%) |
|
Stinging/burning |
28 (4.2%) |
28 (4.3%) |
|
Striae rubrae |
17 (2.5%) |
10 (1.5%) |
|
Telangiectasia |
8 (1.2%) |
12 (1.8%) |
a The denominators for calculating the percentages were the 674 of 709 subjects treated with WINLEVI cream and 656 of 712 subjects treated with vehicle in these trials who had local skin reaction results reported after Day 1.
WINLEVI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients 12-17 years of age and 18 years and older.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The occurrence of overall side effects per subgroups is presented below.
Table 3. Overall Summary of Treatment-Emergent Adverse Events (TEAEs) by Subgroups of Sex, Age, and Race for WINLEVI Arm
|
Adverse Events |
Sex |
Race |
Age (years) |
|||
|---|---|---|---|---|---|---|
|
Male |
Female |
White |
All Other |
12-17 |
≥18 |
|
|
All TEAEs |
27 (10.5) |
55 (11.9) |
77 (11.8) |
5 (7.5) |
34 (10.8) |
45 (11.5) |
|
TEAEs Related to Study Drug |
4 (1.6) |
8 (1.7) |
11 (1.7) |
1 (1.5) |
5 (1.6) |
7 (1.8) |
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved WINLEVI based on evidence from two clinical trials (Trial 1/NCT02608450 and Trial 2/NCT02608476) of 1440 patients with acne vulgaris. The trials were conducted at 99 sites in the United States, Poland, Romania, Bulgaria, Ukraine, Georgia, and Serbia.
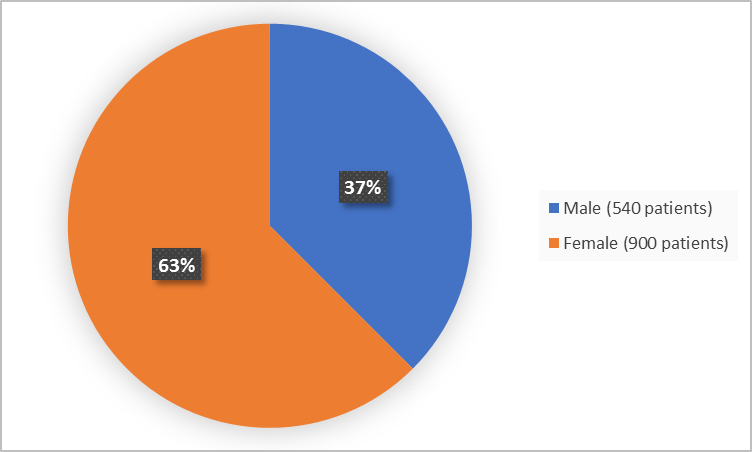
Figure 1 summarizes how many males and females were in the clinical trials.
Figure 1. Demographics by Sex
FDA Review
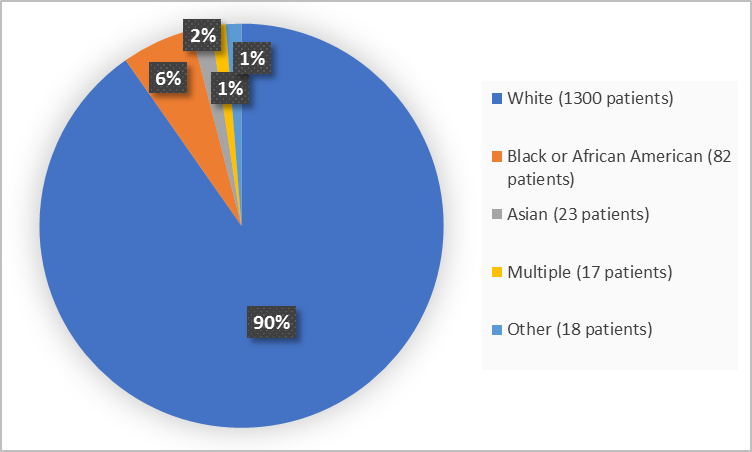
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race
FDA Review
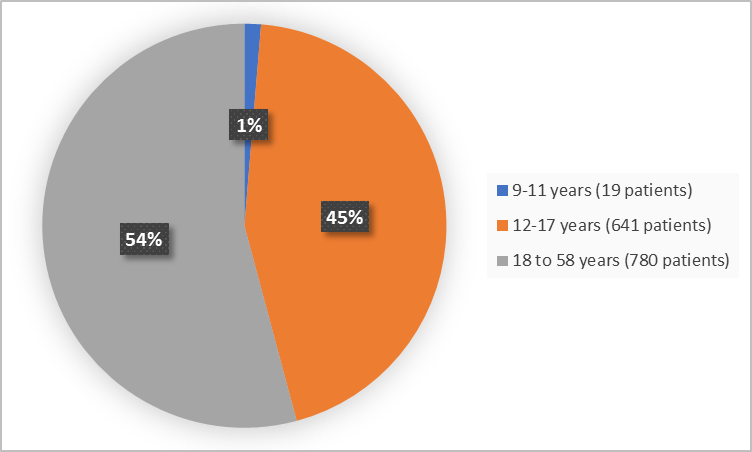
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Demographics by Age
FDA Review
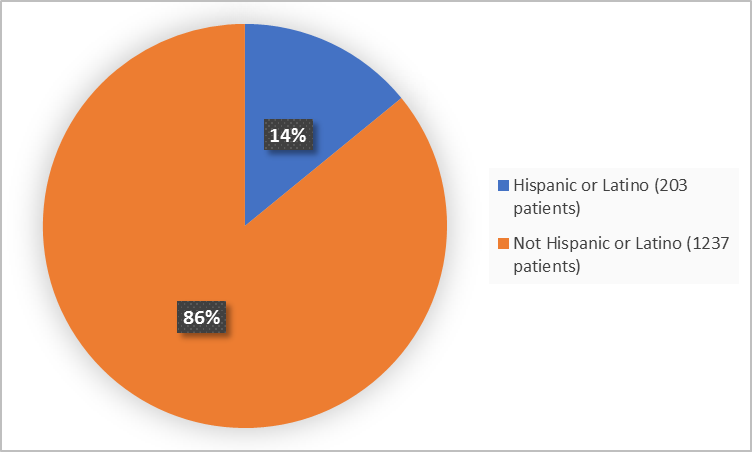
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Baseline Demographics by Ethnicity
FDA Review
Who participated in the trials?
The table below summarizes demographics of the Intent-to-Treat (ITT) population .
Table 4. Demographics of ITT Population
|
Demographic Category |
Trial 1 |
Trial 2 |
Total |
|---|---|---|---|
|
Sex |
|||
|
Male |
272 (38.4) |
268 (36.6) |
540 (37.5) |
|
Female |
436 (61.6) |
464 (63.4) |
900 (62.5) |
|
Race |
|||
|
White |
595 (84) |
705 (96.3) |
1300 (90.3) |
|
Black or African American |
69 (9.7) |
13 (1.8) |
82 (5.7) |
|
Asian |
19 (2.7) |
4 (0.5) |
23 (1.6) |
|
Multiple |
16 (2.3) |
1 (0.1) |
17 (1.2) |
|
Other |
9 (1.3) |
9 (1.2) |
18 (1.2) |
|
Age (years) |
|||
|
Median |
18.0 |
18.0 |
18.0 |
|
Range |
9 to 58 |
10 to 50 |
9 to 58 |
|
Age Categories (years) |
|||
|
9 to 11 |
16 (2.3) |
3 (0.4) |
19 (1.3) |
|
12 to 17 |
300 (42.4) |
341 (46.6) |
641 (44.5) |
|
18 to 58 |
392 (55.4) |
388 (53) |
780 (54.2) |
|
Ethnicity |
|||
|
Hispanic or Latino |
174 (24.6) |
29 (4) |
203 (14.1) |
|
Not Hispanic or Latino |
534 (75.4) |
703 (96) |
1237 (85.9) |
|
Region |
|||
|
USA |
502 (80) |
93 (12.7) |
595 (41.3) |
|
Europe |
206 (20) |
639 (87.3) |
845 (58.7) |
Adapted from FDA Review
How were the trials designed?
The benefit and side effects of WINLEVI were evaluated in two clinical trials of patients 9 to 58 years of age with acne vulgaris.
Patients applied WINLEVI or vehicle (placebo) cream twice daily for 12 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The benefit of WINLEVI in comparison to placebo was assessed after 12 weeks of treatment using the Investigator’s Global Assessment (IGA) score that measures the severity of disease (on a scale from 0 to 4) and a decrease in the number of acne lesions.
How were the trials designed?
The safety and efficacy of WINLEVI were established in 2 clinical trials.
Both trials were randomized, double-blind, placebo-controlled clinical trials designed to evaluate WINLEVI in patients 9 years and older with facial acne vulgaris. Patients were randomized to receive either WINLEVI or vehicle cream twice daily to affected areas.
Efficacy was assessed at Week 12 by the proportion of patients in each treatment group with at least a 2-point reduction in IGA compared to baseline and an IGA score of 0 (clear) or 1 (almost clear), absolute change and percent change from baseline in non-inflammatory and inflammatory lesions.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.

![Winlevi Figure 5. IGA Success at Week 12 by Sex, Race, and Age for Trial 1 [ITT] Table summarizes efficacy results by subgroup in the trial.](https://public4.pagefreezer.com:443/content/FDA/16-06-2022T13:39/https://www.fda.gov/files/Winlevi%20DTS%20Figure%205.png)
![Winlevi Figure 6. IGA Success at Week 12 by Sex, Race, and Age for Trial 2 [ITT] Table summarizes efficacy results by subgroup in the trial.](https://public4.pagefreezer.com:443/content/FDA/16-06-2022T13:39/https://www.fda.gov/files/Winlevi%20DTS%20Figure%206.png)