Drug Trials Snapshot: BENZNIDAZOLE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to BENZNIDAZOLE Prescribing Information for complete information.
BENZNIDAZOLE
Exeltis USA
Approval date: August 29, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BENZNIDAZOLE is used to treat Chagas disease in children 2 to 12 years of age.
Chagas disease is an infectious disease caused by a parasite (Trypanosoma [T.] cruzi). After years of infection, the parasites may cause serious and sometimes deadly heart conditions as well as serious nerve damage and digestion problems.
BENZNIDAZOLE was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
How is this drug used?
BENZNIDAZOLE is a tablet taken by mouth twice a day for 60 days. Total daily dose is determined by the child’s weight.
What are the benefits of this drug?
The benefit was measured by the number of patients who lost antibodies to T. cruzi parasite (test change from positive to negative antibodies, called negative seroconversion). In one trial about 60% of patients who received BENZNIDAZOLE had an antibody test change from positive to negative in comparison to 14% of those who took placebo. In the second trial about 55% of patients who received BENZNIDAZOLE had an antibody test change from positive to negative in comparison to 5% who received placebo.
Another trial will be performed to confirm the clinical benefit of BENZNIDAZOLE.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results conducted at the end of follow-up for each trial separately based on the seroconversion rates measured by nonconventional assays.
Table 1. Nonconventional ELISAa Serologic Status at End-of-Follow-Up (mITT populationb)
|
BENZNIDAZOLE |
Placebo | Difference |
|---|---|---|---|
Trial 1 | N=40 | N=37 |
|
Seronegative | 24 (60.0) | 5 (13.5) | 46.5 (24.5, 64.4) |
Seropositive | 15 | 29 |
|
Missing | 1 | 3 |
|
Trial 2 | N=64 | N=65 |
|
Seronegative | 35 (54.7) | 3 (4.6) | 50.1 (35.8, 63.4) |
Seropositive | 23 | 51 |
|
Missing | 6 | 11 |
|
aEnzymatic-linked immunosorbent assay (F29 ELISA in Trial 1 and AT chemiluminescence-ELISA in Trial 2); the F29 and AT antigens represent antigens from the flagellum of T. cruzi parasites.
bModified intent to treat (mITT) population includes subjects who are positive for the assay at baseline;
cExact confidence intervals presented.
BENZNIDAZOLE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trials that looked at the benefit of BENZNIDAZOLE were too small to determine if there were any differences in sex, and age subgroups. Race data were not collected.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Tables 2-4 summarize efficacy results of seronegative conversion by sex and age for each trial separately.
Due to the small sample size, these exploratory analyses should be interpreted with caution.
Table 2. Serologic Response at Month 48 by Sex using Chemiluminescent Enzymatic Immunoassay (F29) (mITT)-Trial 1
| Sex | BENZNIDAZOLE | Placebo |
|---|---|---|
| Female N=42, n (%) | ||
| Negative | 15 (62.5) | 1 (5.7) |
| Positive | 8 | 16 |
| Missing | 1 | 1 |
| Male N=35, n (%) | ||
| Negative | 9 (56.3) | 4 (21.1) |
| Positive | 7 | 13 |
| Missing | 0 | 2 |
Table 3. Serologic response at Month 36 by Sex using Chemiluminescent Enzymatic Immunoassay (CLEIA) (ITT)-Trial 2
| Sex | BENZNIDAZOLE | Placebo |
|---|---|---|
| Female N=53, n (%) | ||
| Negative | 15 (57.7) | 2 (7.4) |
| Positive | 11 | 25 |
| Male N=76, n (%) | ||
| Negative | 20 (53.6) | 1 (2.6) |
| Positive | 18 | 37 |
Table 4. Serologic response at Month 36 by Age Group Chemiluminescent Enzymatic Immunoassay (CLEIA) (ITT)-Trial 2
| Age Group | BENZNIDAZOLE | Placebo |
|---|---|---|
| 7-9, N=46, n (%) | ||
| Negative | 16 (66.67) | 1 (0.0) |
| Positive | 8 | 21 |
| 10-12, N =83, n (%) | ||
| Negative | 19 (47.50) | 2 (4.65) |
| Positive | 22 | 41 |
Adapted from FDA Statistical review
What are the possible side effects?
BENZNIDAZOLE can cause serious reactions including allergic skin reactions, such as rashes, nerve damage (peripheral neuropathy) and diminished bone marrow function (neutropenia, thrombocytopenia).
The most common side effects of BENZNIDAZOLE are stomach ache, rash, weight loss, headache, nausea, vomiting, low white blood cell count (neutropenia), allergic skin reaction, skin itchiness, and decreased appetite.
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions that occurred in patients in the Trial 1 are summarized in the table below. Adverse reactions that occurred in patients in Trials 2 and 3 are described below.
Table 5. Adverse Reactions in Occurring in Pediatric Patients with Chagas Disease aged 6 to 12 Years -Trial 1
| Body System | Adverse Reaction | BENZNIDAZOLE N=55 n(%) | Placebo N=51 n(%) |
|---|---|---|---|
| Gastrointestinal | Abdominal pain | 14 (25) | 4 (8) |
| Weight decrease | 7 (13) | 1 (2) | |
| Nausea | 3 (5) | 1 (2) | |
| Vomiting | 3 (5) | 0 | |
| Diarrhea | 2 (4) | 0 | |
| Decreased appetite | 3 (5) | 0 | |
| Skin and subcutaneous tissue | Rash | 9 (16) | 0 |
| Metabolism/Laboratory | Transaminases increased | 3 (5) | 0 |
| Nervous system Disorders | Dizziness | 2 (4) | 2 (4) |
| Peripheral neuropathy | 1 (2) | 0 | |
| Tremor | 1 (2) | 0 |
In Trial 2, skin lesions were reported in 7 of 64 (11%) pediatric patients treated with BENZNIDAZOLE and in 2 of 65 patients receiving placebo. Adverse reactions reported in fewer than 5% of BENZNIDAZOLE -treated patients included nausea, anorexia, headache, stomach ache and arthralgia. |
In a subset of 19 pediatric patients 2 to 6 years of age treated with BENZNIDAZOLE in Trial 3, 6 patients (32%) had the following adverse reactions: rash, leukopenia, urticaria, eosinophilia, decreased appetite, and neutropenia. These adverse reactions were similar to those observed in the overall population of 37 patients. |
BENZNIDAZOLE Prescribing Information
Were there any differences in side effects among sex, race and age?
The trials that looked at the side effects of BENZNIDAZOLE were too small to determine if there were any differences in sex, and age subgroups. Race data were not collected.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events during the clinical trial by sex, and age subgroups. Due to the small sample size, these exploratory analyses should be interpreted with caution.
Table 6. Subgroup Analysis of Trial 1 – Adverse Events by Sex and Age
| Subgroup | BENZNIDAZOLE N=55 | Placebo N=51 | Risk Difference (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Total, N | n (%) | Total, N | ||
| Overall | 33 (60.0) | 55 | 14 (27.5) | 51 | 32.55 (14.73, 50.37) |
| SEX | |||||
| Male | 12 (48.0) | 25 | 6 (24.0) | 25 | 24.00 (-1.76, 49.76) |
| Female | 21 (70.0) | 30 | 8 (30.8) | 26 | 39.23 (15.07,63.39) |
| AGE | |||||
| 14 (58.3) | 24 | 4 (19.0) | 21 | 39.29 (13.38, 65.19) | |
| >= 10 years | 19 (61.3) | 31 | 10 (33.3) | 30 | 27.96 (3.90, 52.01) |
Table 7. Subgroup Analysis of Trial 2 - Adverse Events by Sex and Age
| Subgroup | BENZNIDAZOLE N=64 | Placebo N=65 | Risk Difference (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Total, N | n (%) | Total, N | ||
| Overall | 7 (10.9) | 64 | 2 (3.1) | 65 | 7.86 (-0.86, 16.58) |
| SEX | |||||
| Male | 5 (13.2) | 38 | 0 | 38 | 13.16 (2.41, 23.91) |
| Female | 2 (7.7) | 26 | 2 (7.4) | 27 | 0.28 (-13.95, 14.51) |
| AGE | |||||
| 10> | 1 (4.2) | 24 | 0 | 22 | 4.17 (-3.83, 12.16) |
| >= 10 years | 6 (15.00) | 40 | 2 (4.7) | 43 | 10.35 (-2.38, 23.08) |
FDA Clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BENZNIDAZOLE based primarily on evidence from the two published clinical trials of 235 children with Chagas disease. Trial 1 was conducted from 1991-1995 in Argentina and Trial 2 from 1991-1995 in Brazil. Demographics of these two trials are presented in the Table 8 and Figures 1 and 2 below.
The FDA also considered data from an additional published trial that provided dosing and safety data for children with Chagas disease as young as 2 years of age. The trial was conducted from 2008-2010 in Argentina. Demographics of this trial are presented in the Table 9.
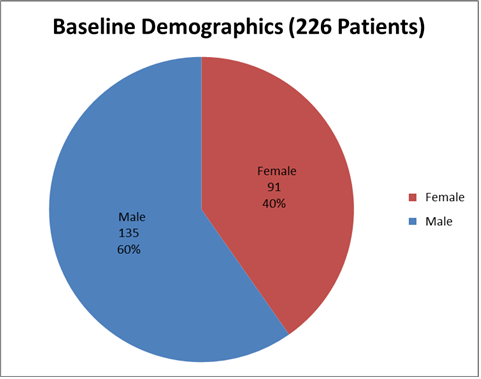
Figure 1 summarizes by sex how many children were in the clinical trials 1 and 2.
Figure 1. Baseline Demographics by Sex
Adapted from FDA Clinical review
Demographics by Race
Data not collected.
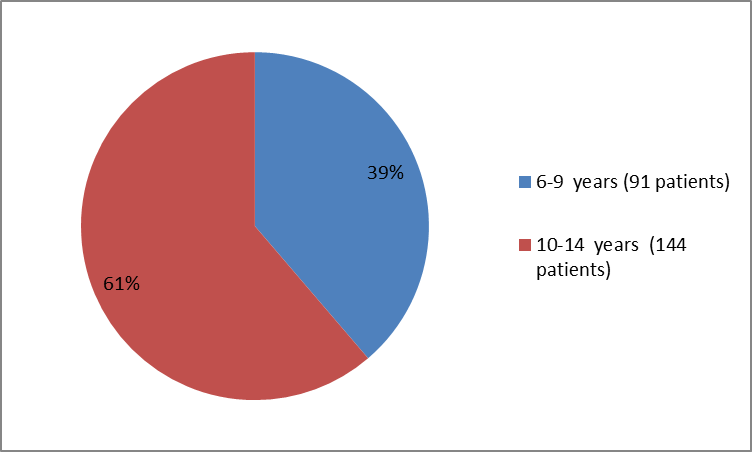
Figure 2 summarizes how many patients of a certain age were enrolled in the clinical trials.
Figure 2. Baseline Demographics by Age
Adapted from FDA Clinical review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials that supported safety and efficacy in children 6-12 years of age. Race and ethnicity were not collected.
Table 8. Baseline Demographics of Patients in the Clinical Trials 1 and 2
| Demographic Parameters | Trial 1 (N=106) n (%) | Trial 2 (N=129) n (%) | Total (N=235) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 50 | 76 | 126 |
| Female | 56 | 53 | 109 |
| Age (years) | |||
| Min., Max. | 6,14 | 7,12 | 6,14 |
| Age Group | |||
| 10> | 45 | 46 | 91 |
| ≥10 years | 61 | 83 | 144 |
Adapted from FDA Clinical review
The table below summarizes demographics of patients in the clinical trial that supported safety and extrapolation of efficacy in children 2-6 years of age.
Table 9. Baseline Demographics of Patients in the Clinical Trial 3
| Demographic Parameters | Trial 3 (N=37) n (%) |
|---|---|
| Sex | |
| Male | 17 (46) |
| Female | 20 (44) |
| Age (years) | |
| Median, (Min., Max.) | 6 (2,12) |
| Age Group | |
| 2-6 years | 19 |
| ≥6 years | 18 |
Adapted from FDA Clinical review
How were the trials designed?
There were two trials that provided data for assessment of benefit and side effects of BENZNIDAZOLE.
In Trial 1, children 6 to 12 years of age with chronic Chagas disease and without symptoms of disease were randomly treated with either BENZNIDAZOLE or placebo tablets twice daily for 60 days. Patients were followed for 4 years. Neither the patients nor the investigators knew which treatment was given until the trial ended.
In Trial 2, children 7 to 12 years of age with chronic Chagas disease were randomly treated with either BENZNIDAZOLE or placebo tablets twice daily for 60 days. Patients were followed for 3 years. Neither the patients nor the investigators knew which treatment was given until the trial ended.
The benefit of BENZNIDAZOLE was evaluated at the end of follow-up by measuring the presence of antibodies in the blood.
One additional trial, Trial 3 (NCT00699387) provided data for assessment of safety in children 2 to 6 years of age. In this trial all children were treated with BENZNIDAZOLE twice daily for 60 days.
How were the trials designed?
The safety and efficacy of BENZNIDAZOLE were established in two, randomized, double blind, placebo-controlled trials in children with chronic intermediate Chagas disease.
Trial 1 was conducted from 1991-1995 in Argentina. Children 6 to 12 years of age received either BENZNIDAZOLE 5/mg/kg/day or placebo twice daily for 60 days and were followed for 4 years.
Trial 2 was conducted from 1991-1995 in Brazil. Children 7 to 12 years of age received either BENZNIDAZOLE 7.5mg/kg/day twice daily or placebo for 60 days and were followed for 3 years.
Both trials measured antibodies by conventional and nonconventional assays.
In an open label, pharmacokinetic Trial 3, that supported safety and extrapolation of efficacy in children 2-6 years of age, children 2-12 years received BENZNIDAZOLE 5-8mg/kg/ twice a day for 60 days and were followed up for 10 weeks.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.