Drug Trials Snapshot: BRINEURA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to BRINEURA Prescribing Information for complete information.
BRINEURA (cerliponase alfa)
brin ure' a
BioMarin Pharmaceutical Inc.
Approval date: April 27, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BRINEURA is a drug that slows loss of walking ability (ambulation) in symptomatic patients with a specific form of Batten disease. It is to be used in patients 3 years of age and older.
This form of Batten disease is known as late infantile neuronal ceroid lipofuscinosis type 2 (CLN2). CLN2 is a rare, inherited, childhood disease that is characterized by the deficiency of tripeptidyl peptidase-1 (TPP1) enzyme, primarily affecting the central nervous system. The lack of enzyme causes progressive problems with vision, movement, and thinking ability and premature death.
How is this drug used?
BRINEURA is given by a health care professional through a special infusion system once every other week directly into the fluid inside the brain chamber. The infusion system includes a syringe pump, a reservoir underneath the skin and a catheter. This method of administration is known as an intraventricular infusion. It takes approximately 4.5 hours to complete the infusion.
What are the benefits of this drug?
In the trial, BRINEURA-treated patients demonstrated a delay in decline of walking ability compared to untreated patients from a natural history cohort.
To further assess efficacy, the 22 BRINEURA-treated patients were matched based on age, genotype, and baseline Motor domain score to the untreated patients. Information on the 17 matched pairs is in Table 2.
Table 2. Proportion of Matched Symptomatic Pediatric Patients with CLN2 Disease without Decline* in the BRINEURA Single-Arm Clinical Study with Extension and in the Natural History Cohort assessed at Weeks 48, 72, and 96
|
Time Point/Period |
Natural History Cohort (N=17) |
BRINEURA-Treated |
Difference |
Odds Ratio*** |
|---|---|---|---|---|
|
|
n (%) |
% (95% CI**) |
OR (95% CI) |
|
|
Follow-up through |
13 (76) |
16 (94) |
18% (-19, 51) |
4 (0.4, 200) |
|
Follow-up through |
11 (65) |
16 (94) |
29% (-7, 61) |
5.9 (0.7, 250) |
|
Follow-up through |
6 (35) |
16 (94) |
59% (24, 83) |
11 (1.6, 500) |
*Decline is defined as an unreversed (sustained) 2-category decline or unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale.
** Exact confidence interval for risk difference (Santner and Snell)
***Based on McNemar’s Exact test
Matched on baseline age at time of screening within 3 months, genotype (0, 1, or 2 key mutations), and baseline Motor domain CLN2 score at time of screening.
The Brineura-treated population is based on the full population minus two patients with baseline Motor plus Language CLN2 score = 6.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of BRINEURA was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
There were insufficient numbers of patients to perform any subpopulation analyses.
What are the possible side effects?
BRINEURA may cause serious adverse reactions including device-related complications (leakage and infection), low-blood pressure and heart rhythm problems, and allergic reactions.
The side effects that occurred in at least 50% of the patients were fever, changes in ECG (electrical heart beat tracing), decreased protein content in the brain fluid, vomiting, and seizures.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with BRINEURA.
Table 3. Adverse Reactions Reported in ≥ 8% of Symptomatic Pediatric Patients with CLN2 Disease in the BRINEURA Single-Arm Clinical Study with Extension at Week 96
|
Adverse Reaction |
Patients Treated with BRINEURA |
|---|---|
|
Pyrexia* |
17 (71) |
|
ECG abnormalities† |
17 (71) |
|
CSF protein decreased |
17 (71) |
|
Vomiting |
15 (63) |
|
Seizures‡ |
12 (50) |
|
Hypersensitivity§ |
11(46) |
|
CSF protein increased |
5 (21) |
|
Hematoma |
5 (21) |
|
Headache |
4 (17) |
|
Irritability |
4 (17) |
|
Pleocytosis |
4 (17) |
|
Device-related infection¶ |
2 (8) |
|
Bradycardia |
2 (8) |
|
Feeling jittery |
2 (8) |
|
Hypotension |
2 (8) |
*Pyrexia includes: pyrexia and increased body temperature
†ECG abnormalities include: non-specific repolarization abnormality, notched QRS, ST segment elevation, biphasic T wave abnormality, supraventricular extrasystoles, bradycardia, sinus tachycardia, and intraventricular conduction delay
‡Seizures include: atonic, generalized tonic-clonic, focal, and absence.
§Hypersensitivity includes: immune reactions and signs and symptoms observed concomitantly with hypersensitivity reactions including pyrexia, vomiting, pleocytosis or irritability
¶Device-related infections include: Propionibacterium acnes and Staphylococcus epidermidis
Were there any differences in side effects among sex, race and age?
The trial that looked at the side effects of BRINEURA was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
There were insufficient numbers of patients to perform any subpopulation analyses
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
There was one clinical trial of 24 patients with CLN2 disease. The trial was conducted in the United States, United Kingdom, Germany and Italy.
These 24 patients represent the population that was used to assess the side effects of BRINEURA (called the safety population) and is presented in the figures below.
Some of these patients (total of 22 patients) represent the population that was used to establish the benefit of BRINEURA. These patients are presented in Table 5 under MORE INFO section.
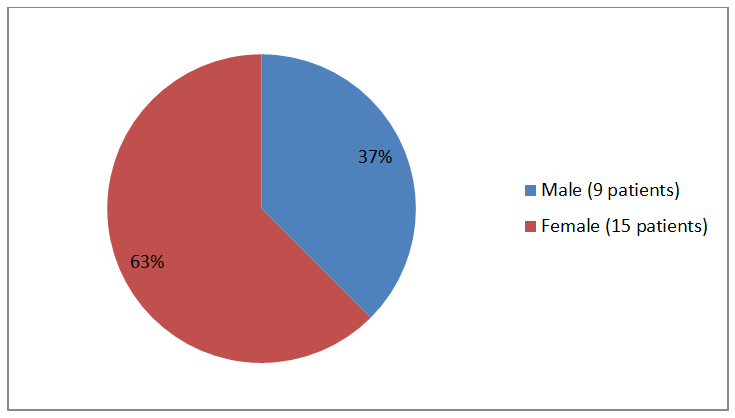
The figure below summarizes patients who were enrolled in the trial by sex.
Figure 1. Baseline Demographics by Sex (safety population)
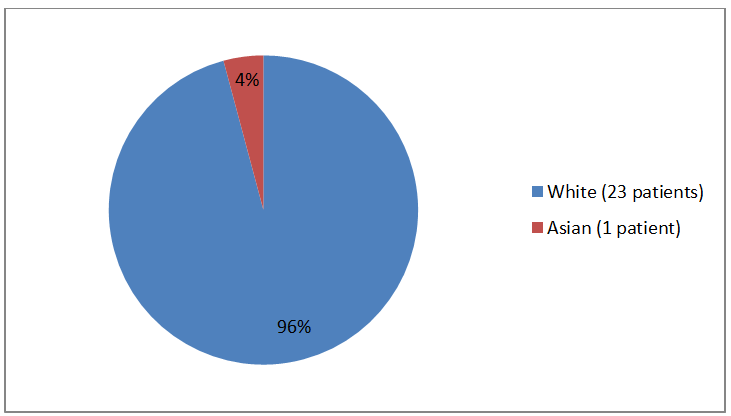
Figure 2 and Table 1 below summarize the percentage of enrolled patients by race.
Figure 2. Baseline Demographics by Race (safety population)
Clinical Trial Data
Table 1: Baseline Demographics by Race
|
Race |
Number of Patients |
Percentage |
|---|---|---|
|
White |
23 |
96 |
|
Asian |
1 |
4 |
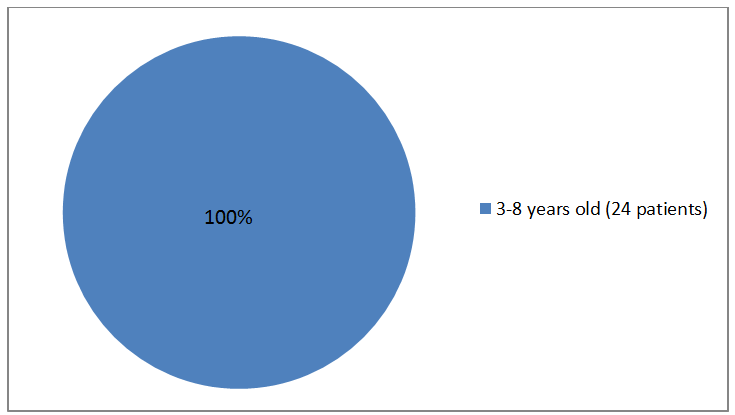
Figure 3 summarizes the percentage of patients by age group in the trial.
Figure 3. Baseline Demographics by Age (safety population)
Clinical Trial Data
Who participated in the trials?
Tables below summarize demographics of patients in the clinical trial.
Table 4. Baseline Demographics of Patients in the Clinical Trial (safety population)
|
Demographic Parameters |
Trial 1 |
|---|---|
|
Sex |
|
|
Male |
9 (37) |
|
Female |
15 (63) |
|
Age |
|
|
Mean years (SD) |
4.9 (1.3) |
|
Median (years) |
4.7 |
|
Min, max (years) |
3.1,8.9 |
|
Race |
|
|
White |
23 (96) |
|
Asian |
1 (4) |
|
Ethnicity |
|
|
Hispanic or Latino |
1 (4) |
|
Not Hispanic or Latino |
23 (96) |
Clinical Trial Data
Table 5. Baseline Demographics of Enrolled Patients in the Clinical Trial (efficacy population)
|
Demographic Parameters |
Trial 1 |
|---|---|
|
Sex |
|
|
Male |
7 (32) |
|
Female |
15 (68) |
|
Age |
|
|
Mean years (SD) |
4.9 (1.3) |
|
Median (years) |
4.7 |
|
Min, max (years) |
3.6,8.9 |
|
Race |
|
|
White |
21 (96) |
|
Asian |
1 (4) |
|
Ethnicity |
|
|
Hispanic or Latino |
1 (4) |
|
Not Hispanic or Latino |
21 (96) |
Clinical Trial Data
How were the trials designed?
There was one trial that evaluated the benefit and side effects of BRINEURA. The trial enrolled pediatric patients with late infantile neuronal CLN2 disease.
BRINEURA-treated patients were compared to untreated patients from a natural history cohort by assessing disease progression through Week 96 of treatment. The investigators measured the loss of ability to walk or crawl using the Motor domain of the CLN2 Clinical Rating Scale. Scores from the Motor domain of the scale range from 3 (grossly normal) to 0 (profoundly impaired.)
How were the trials designed?
The safety and efficacy of BRINEURA were evaluated in a single, non-randomized, single-arm multicenter clinical trial that enrolled 24 pediatric patients with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease, confirmed to have TPP1 deficiency. Patients received BRINEURA via intraventricular infusion every two weeks for 48 weeks and continued the treatment during the extension period.
Brineura-treated patients were compared to untreated patients from a natural history cohort in the Motor domain of the CLN2 Clinical Rating Scale over 96 weeks. CLN2 Clinical Rating Scale Motor domain scores ranged from 3 (grossly normal) to 0 (profoundly impaired). Decline was defined as having an unreversed (sustained) 2-category decline or an unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.