Drug Trials Snapshot: CABENUVA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CABENUVA Prescribing Information for complete information.
CABENUVA (cabotegravir and rilpivirine)
(kab' en ue vah)
ViiV Healthcare
Approval date: January 20, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CABENUVA is a 2-drug co-packaged product for the treatment of human immunodeficiency virus-1 (HIV-1) infection in adults who are virologically supressed and need to replace their current HIV-1 medicines.
HIV-1 is the virus that causes acquired immune deficiency syndrome (AIDS).
How is this drug used?
CABENUVA is an injection. Two co-packaged injections (cabotegravir and rilpivirine) are injected by healthcare provider into the muscle of each side of buttocks one time every month.
Before receiving first injection doses of CABENUVA, patients need to take 1 VOCABRIA (cabotegravir) tablet and 1 EDURANT (rilpivirine) tablet once a day for one month to ensure the medications are well-tolerated.
What are the benefits of this drug?
CABENUVA kept HIV-1 viral load at suppressed level.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the primary efficacy endpoint defined as the proportion of patients with plasma HIV-1 RNA greater than or equal to 50 copies/mL at Week 48.
Table 1. Virologic Outcomes of Randomized Treatment in FLAIR and ATLAS Trials at Week 48
|
Virologic Outcomes |
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
CAB plus RPV |
CAR |
CAB plus RPV |
CAR |
|
|
HIV-1 RNA ≥50 copies/mLa |
2% |
2% |
2% |
1% |
|
Treatment Difference |
-0.4% |
0.7% |
||
|
HIV-1 RNA <50 copies/mL |
94% |
93% |
93% |
95% |
|
No virologic data at Week 48 window |
4% |
4% |
6% |
4% |
|
Discontinued due to adverse event or death |
3% |
<1% |
4% |
2% |
|
Discontinued for other reasons |
1% |
4% |
2% |
2% |
|
Missing data during window but on study |
0 |
0 |
0 |
0 |
a Includes patients who discontinued for lack of efficacy and discontinued while not suppressed.
n = Number of subjects in each treatment group, CI = Confidence interval, CAB = Cabotegravir,
RPV = Rilpivirine, CAR = Current antiretroviral regimen.
CABENUVA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: CABENUVA worked similarly in men and women.
- Race: CABENUVA worked similarly in all tested races.
- Age: The majority of patients in the trials were below 65 years of age. Differences in how well CABENUVA worked between those below and above 65 years of age could not be determined.
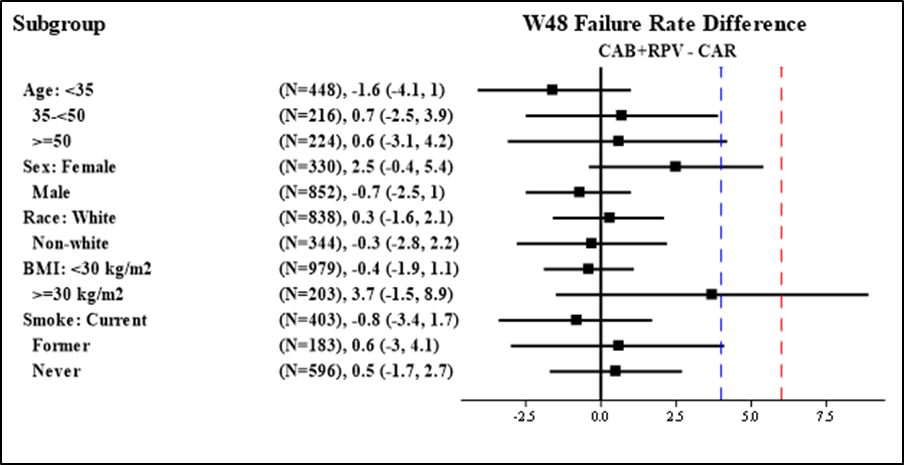
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses of the primary endpoint are presented below.
Figure 5. Subgroup Analyses, Baseline Demographic Characteristics, Trials 1 and 2 Combined
FDA Review
What are the possible side effects?
CABENUVA may cause a serious side effects including allergic and post-injection reactions, an increase of liver enzymes, and depression.
The most common side effect associated with CABENUVA are injection site reactions, fever, fatigue, headache, muscle and joint pain, nausea, sleeping problems, dizziness and rash.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions reported in pooled trials.
Table 2. Adverse Reactionsa (Grades 1 to 4) Reported in at Least 2% of Patients with HIV-1 Infection in the Trials 1 and 2 (Week 48 Pooled Analyses)
|
|
Cabotegravir plus Rilpivirine |
Current Antiretroviral Regimen |
||
|---|---|---|---|---|
|
All Grades |
At Least Grade 2 |
All Grades |
At Least Grade 2 |
|
|
Injection site reactionsb |
83% |
37% |
0 |
0 |
|
Pyrexiac |
8% |
2% |
0 |
0 |
|
Fatigued |
5% |
1% |
<1% |
<1% |
|
Headache |
4% |
<1% |
<1% |
<1% |
|
Musculoskeletal paine |
3% |
1% |
<1% |
0 |
|
Nausea |
3% |
<1% |
1% |
<1% |
|
Sleep disordersf |
2% |
<1% |
<1% |
0 |
|
Dizziness |
2% |
<1% |
<1% |
0 |
|
Rashg |
2% |
<1% |
0 |
0 |
a Adverse reactions defined as “treatment-related” as assessed by the investigator.
b See Injection-Associated Adverse Reactions for additional information.
c Pyrexia: includes pyrexia, feeling hot, chills, influenza-like illness, body temperature increased.
d Fatigue: includes fatigue, malaise, asthenia.
e Musculoskeletal pain: includes musculoskeletal pain, musculoskeletal discomfort, back pain, myalgia, pain in extremity.
f Sleep disorders: includes insomnia, poor quality sleep, somnolence.
g Rash: includes erythema, pruritus, pruritus generalized, purpura, rash, rash- erythematous, generalized, macular.
CABENUVA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all tested races.
- Age: The majority of patients in the trials were below 65 years of age. Differences in the occurrence of side effects between those below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of adverse events by demographic subgroups during maintenance period in pooled trials.
Table 3. Summary of Adverse Events Among Male Patients
|
|
|
ABC/DTG/ |
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
210 (95%) |
175 (80%) |
203 (97%) |
148 (73%) |
413 (96%) |
323 (76%) |
|
Grade 3/4 AE |
28 (13%) |
9 (4%) |
28 (13%) |
17(8%) |
56 (13%) |
26 (6%) |
Table 4. Summary of Adverse Events Among Female Patients
|
|
|
ABC/DTG/ |
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
57 (90%) |
50 (78%) |
91 (92%) |
72 (69%) |
148 (91%) |
122 (73%) |
|
Grade 3/4 AE |
3 (5%) |
2 (3%) |
7 (7%) |
7 (7%) |
10 (6%) |
9 (5%) |
Table 5. Summary of Adverse Events Among White Patients
|
|
|
|
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
205 (95%) |
155 (77%) |
209 (98%) |
150 (72%) |
414 (96%) |
305 (75%) |
|
Grade 3/4 AE |
27 (13%) |
9 (4%) |
25 (12%) |
11 (5%) |
52 (12%) |
20 (5%) |
Table 6. Summary of Adverse Events Among Black or African American Patients
|
|
|
|
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
42 (89%) |
45 (80%) |
54(87%) |
51(66%) |
96 (88%) |
96 (72%) |
|
Grade 3/4 AE |
3 (6%) |
1 (2%) |
6 (10%) |
9 (12%) |
9 (8%) |
10 (8%) |
Table 7. Summary of Adverse Events Among Patients by Age (<50 years old )
|
|
|
ABC/DTG/ |
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
234 (94%) |
201 (79%) |
231 (95%) |
147(69%) |
465 (95%) |
348 (75%) |
|
Grade 3/4 AE |
27 (11%) |
9 (4%) |
30 (12%) |
18 (8%) |
57 (12%) |
27 (6%) |
Table 8. Summary of Adverse Events Among Patients by Age (≥50 years old)
|
|
|
ABC/DTG/ |
|
Current |
|
Current |
|---|---|---|---|---|---|---|
|
Any AE |
33 (100%) |
24 (83%) |
63 (95%) |
73 (76%) |
96 (97%) |
97 (78%) |
|
Grade 3/4 AE |
4 (12%) |
2 (7%) |
5 (8%) |
6 (6%) |
9 (9%) |
8 (6%) |
Abbreviations: 3TC = lamivudine, ABC = abacavir, AE = adverse event, ART = antiretroviral therapy, CAB = cabotegravir, DTG = dolutegravir, RPV = rilpivirine, SAE = serious adverse event
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved CABENUVA based on evidence from two clinical trials (Trial 1/ NCT02938520 and Trial 2/ NCT02951052) which enrolled 1182 adults with HIV-1 infection. Trials were conducted at 223 sites in 24 countries including the United States.
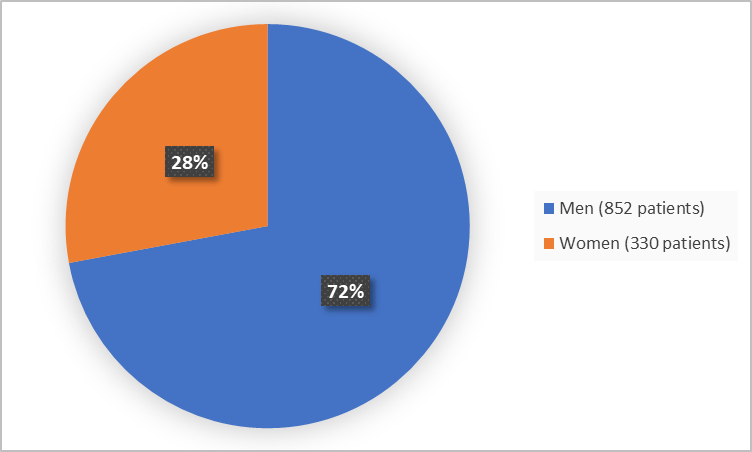
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex
Adapted from FDA Review
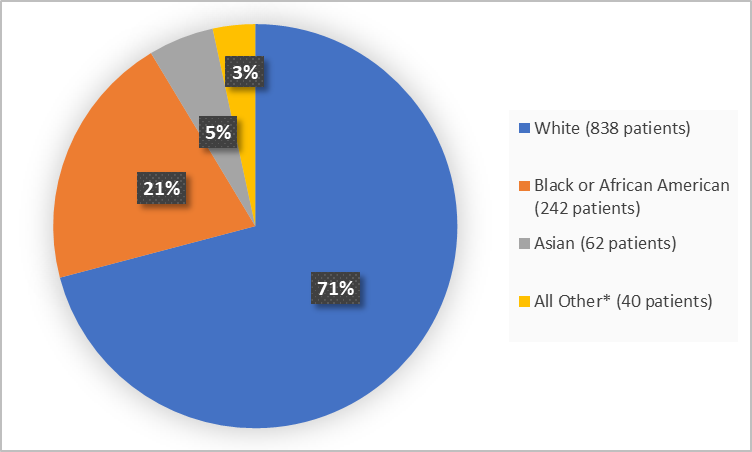
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race
*Includes American Indian or Alaska Native, Multiple, Native Hawaii or Other Pacific Islander and Missing
Adapted from FDA Review
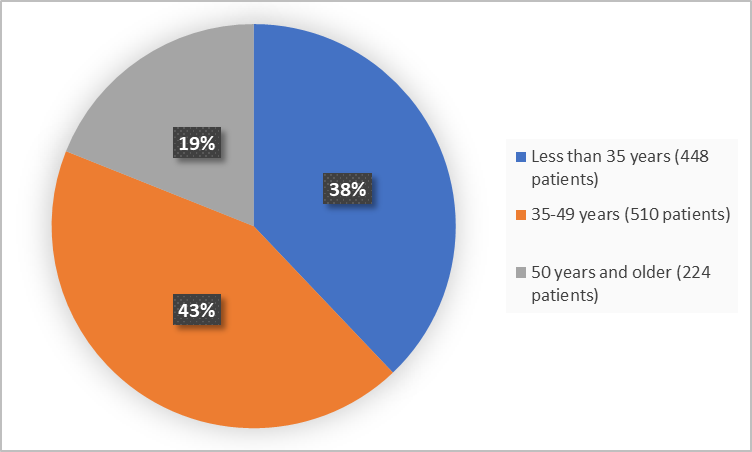
Figure 3 summarizes patients by age in the clinical trials.
Figure 3. Demographics by Age
Adapted from FDA Review
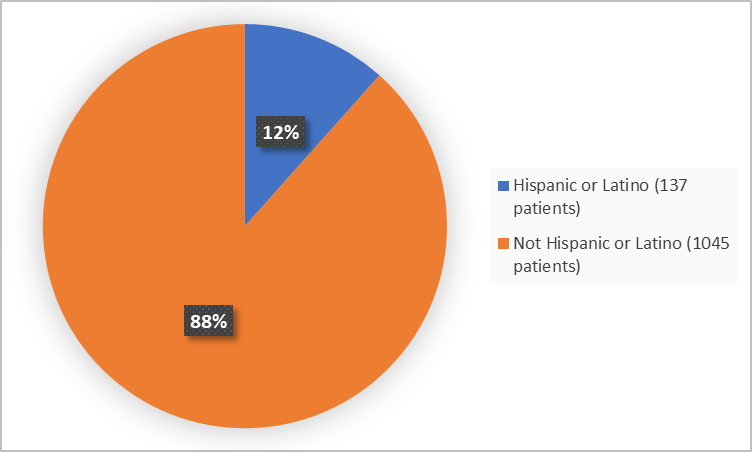
Figure 4 summarizes patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
The table below summarizes demographics in pooled trials.
Table 9. Trials Demographics
|
Demographic Characteristic |
CABENUVA |
Current AVR |
Total |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Men |
429 (73) |
423 (72) |
852 (72) |
|
Women |
162 (27) |
168 (28) |
330 (28) |
|
Race, n (%) |
|||
|
White |
430 (73) |
408 (69) |
838 (71) |
|
Black or African American |
109 (18) |
133 (23) |
242 (20) |
|
Asian |
34 (6) |
28 (5) |
62 (5) |
|
American Indian or Alaska Native |
11 (2) |
14 (2) |
25 (2) |
|
Multiple |
6 (1) |
5 (1) |
11 (1) |
|
Native Hawaii or Other Pacific Islander |
1 (<1) |
1 (<1) |
2 (<1) |
|
Missing |
0 |
2 (<1) |
2 (<1) |
|
Age, years |
|||
|
Median (min, max) |
38 (19, 74) |
38 (18,82) |
38 (18,82) |
|
Age groups, n (%) |
|||
|
<35 years |
223 (38) |
225 (38) |
448 (38) |
|
35-49 years |
269 (46) |
241 (41) |
510 (43) |
|
≥50 years |
99 (17) |
125 (21) |
224 (19) |
|
≥65 years |
8 |
10 |
18 (2) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
63 (11) |
74 (13) |
137 (12) |
|
Not Hispanic or Latino |
528 (89) |
517 (87) |
1045 (88) |
|
Region, n (%) |
|||
|
USA |
107 |
135 |
242 (20) |
|
Canada |
27 |
30 |
57 (5) |
|
Rest of World* |
457 |
426 |
883 (75) |
*Argentina, Australia, Germany, Spain, France, Great Britain, Italia, Japan, South Korea, Mexico, Netherlands, Russia, Sweden and South Africa.
Adapted from FDA Review
How were the trials designed?
CABENUVA was evaluated in two clinical trials that enrolled adults with HIV-1 infection.
In Trial 1, patients who were never treated for the infection before, received an approved therapy for 20 weeks. Those who did well after this treatment (who had HIV-1 RNA less than 50 copies/milliliter) were then randomized to receive either CABENUVA (for the first 4 weeks they received tablets) or to remain on the same therapy for additional 44 weeks. Patients and the health providers knew which treatments have been given.
In Trial 2, patients who were previously successfully treated for the infection (who had HIV-1 RNA less than 50 copies/milliliter), were randomized to receive either CABENUVA (for the first 4 weeks they received tablets) or to remain on the same therapy for additional 44 weeks. Patients and the health providers knew which treatments have been given.
The efficacy of CABENUVA in comparison to control treatment was assessed after 48 of treatment by comparing the proportions of patients with detectable levels of HIV-1 RNA in the blood (at least 50 copies/milliliter or more).
How were the trials designed?
FDA approved CABENUVA based on data from two randomized, multicenter, active-controlled, parallel-arm, open-label, non-inferiority trials.
Trial 1 enrolled HIV-1–infected, antiretroviral treatment (ART)-naive patients who were virologically suppressed. They were randomized (1:1) to receive either a cabotegravir plus rilpivirine regimen or remain on the current antiretroviral regimen. Patients randomized to receive cabotegravir plus rilpivirine initiated treatment with daily oral lead-in dosing with one 30-mg VOCABRIA (cabotegravir) tablet plus one 25-mg EDURANT (rilpivirine) tablet for at least 4 weeks followed by monthly injections with CABENUVA for an additional 44 weeks.
Trial 2 enrolled HIV-1–infected, ART-experienced, virologically-suppressed who were randomized and received either a cabotegravir plus rilpivirine regimen or remained on their current antiretroviral regimen. Patients randomized to receive cabotegravir plus rilpivirine initiated treatment with daily oral lead-in dosing with one 30-mg VOCABRIA (cabotegravir) tablet plus one 25-mg EDURANT (rilpivirine) tablet for at least 4 weeks followed by monthly injections with CABENUVA for an additional 44 weeks.
The primary endpoint in both trials was the proportion of subjects with plasma HIV-1 RNA greater than or equal to 50 copies/mL at Week 48.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.