Drug Trials Snapshot: Cholbam (peroxisomal disorders)
For treatment of peroxisomal disorders, including Zellweger spectrum disorders
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CHOLBAM Prescribing Information for complete information.
CHOLBAM (cholic acid)
(KOHL-bam)
Asklepion Pharmaceuticals, LLC
Approval date: March 17, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CHOLBAM is a treatment for children and adults who have a rare condition called bile acid synthesis disorders. This happens when infants are born with defects that result in low bile acid levels. This can lead to problems with growth and serious damage of the liver and of the nervous system. The liver normally makes bile acids which are needed to digest fat and absorb Vitamins A, D, E, and K.
CHOLBAM was studied for the treatment of a type of these conditions called peroxisomal disorders (PDs), including Zellweger Spectrum Disorders. It is intended for patients who have problems with their liver, have too much fat in their feces, or complications resulting from the inability to absorb certain vitamins.
CHOLBAM was also studied for the treatment of what is called bile acid synthesis disorders due to single enzyme defects (SEDs). This Snapshot discusses CHOLBAM for PDs. A separate Snapshot discusses CHOLBAM for the treatment of patients with SEDs.
How is this drug used?
CHOLBAM is a capsule taken by mouth once or twice each day.
What are the benefits of this drug?
In the trials that supported the FDA approval of CHOLBAM, 46% of patients with rare bile acid synthesis disorders had improvement in body weight and in liver tests that are expected to be related to less liver damage. Forty-two percent of patients survived more than three years.
What are the benefits of this drug (results of trials used to assess efficacy)?
Response to CHOLBAM treatment was assessed by the following laboratory criteria:
- Alanine transaminase (ALT) or aspartate transaminase (AST) values reduced to less than 50 U/L, or baseline levels reduced by 80%;
- Total bilirubin values reduced to less than or equal to 1mg/dL; and
- No evidence of cholestasis (bile not being released from the liver) on liver biopsy;
And the following clinical criteria:
- Body weight increased by 10% or stable at greater than the 50th percentile; and
- Survival for greater than three years on treatment or alive at the end of Trial 2
CHOLBAM responders were defined as patients who either:
- Met at least two laboratory criteria and were alive at the last follow-up or
- Met at least one laboratory criterion, had increased body weight and were alive at the last follow-up
Overall, 11 of 24 patients (46%) were responders. The table below breaks down responders by defect type.
Table 2. Response to CHOLBAM Treatment by Type of Peroxisomal Disorder including Zellweger Spectrum Disorders
| Peroxisomal Disorder | Responders/Number Treated (%) |
|---|---|
| Neonatal Adrenoleukodystrophy | 3/6 (50) |
| Generalized Peroxisomal Disorder | 1/1 (100) |
| Refsum Disease | 3/4 (75) |
| Zellweger Syndrome | 3/8 (38) |
| Peroxisomal Disorder, Type Unknown | 1/5 (20) |
Source: CHOLBAM Package Insert, Section 14, Table 5
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The studies that looked at benefit of CHOLBAM were too small to determine if there were differences in sex, race, and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Table 3. Subgroup Analysis of Primary Endpoint in Clinical Trials, Peroxisomal Disorders
| Subgroup | Proportion of Responders | 95 % CI |
|---|---|---|
| Overall Response/All Patients | 0.46 | (0.44, 0.48) |
| Sex | ||
| Male | 0.25 | (0.23, 0.27) |
| Female | 0.88 | (0.85, 0.91) |
| Age Group | ||
| <17 | 0.46 | (0.44, 0.48) |
| >=17 | - | - |
| >=65 years | - | - |
| >=75 years | - | - |
| Race | ||
| White | 0.45 | (0.43, 0.47) |
| Black or African American | - | - |
| Asian | - | - |
| American Indian or Alaska Native | - | - |
| Native Hawaiian or Other Pacific Islander | - | - |
| Unknown | 0.5 | (0.38, 0.62) |
Source: From Clinical Reviewer
What are the possible side effects?
The most common side effect in patients treated with CHOLBAM was diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the most common adverse reactions in the two trials. These include reactions that occurred in patients with either Single Enzyme Disorders or Peroxisomal Disorders.
Table 4. Most Common Adverse Reaction in Trials 1 and 2
Adverse Reaction |
Trial 1 | Trial 2 | Overall (%) |
|---|---|---|---|
| Diarrhea | 1 | 2* | 3 (2) |
| Reflux Esophagitis | 1 | 0 | 1 (1) |
| Malaise | 1 | 0 | 1 (1) |
| Jaundice | 1 | 0 | 1 (1) |
| Skin lesion | 1 | 0 | 1 (1) |
| Nausea | 0 | 1* | 1 (1) |
| Abdominal Pain | 0 | 1* | 1 (1) |
| Intestinal Polyp | 0 | 1* | 1 (1) |
| Urinary Tract Infection | 0 | 1* | 1 (1) |
| Peripheral Neuropathy | 0 | 1 | 1 (1) |
*Adverse Reactions that occurred in new patients
Source: CHOLBAM Prescribing Information, Section 6, Table 3
Were there any differences in side effects among sex, race and age?
The studies that looked at the side effects of CHOLBAM were too small to determine if there were differences in sex, race, and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes the percentage of patients in each subgroup who experienced at least one treatment-emergent adverse event. Patients represented in the table are those with PD who received CHOLBAM.
Table 5. Subgroup Analysis of Adverse Events that Occurred During Treatment (Safety Population)
| Subgroup | Treatment Group (n= 31) |
|
|---|---|---|
| n (%) | Total, N | |
| Sex | ||
| Male | 13 (68%) | 19 |
| Female | 9 (75%) | 12 |
| Age Group | ||
| <17 | 22 (71)% | 31 |
| >=17 | 0 | |
| >=65 years | 0 | |
| >=75 years | 0 | |
| Race | ||
| White | 15 (65)% | 23 |
| Black or African American | 1 (100%) | 1 |
| Asian | 0 | |
| American Indian or Alaska Native | 0 | |
| Native Hawaiian or Other Pacific Islander | 0 | |
| Other | 6 (86%) | 7 |
| Ethnicity | ||
| Hispanic or Latino | 3 (100%) | 3 |
| Not Hispanic or Latino | 17 (68%) | 25 |
| Unknown | 2 (67%) | 3 |
Source: From Clinical Reviewer
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved CHOLBAM based on a main trial (called Trial 1) that took place over 18 years and a second trial (called Trial 2) that continued treatment for many patients enrolled in Trial 1. Trial 2 is known as an extension trial.
The first trial included 29 patients with PDs. The extension trial included 10 patients who continued receiving treatment, as well as another two patients who began treatment with CHOLBAM. Information on the benefit of CHOLBAM was available for 24 patients.
The trials were conducted in North America, South America, Europe, and Asia.
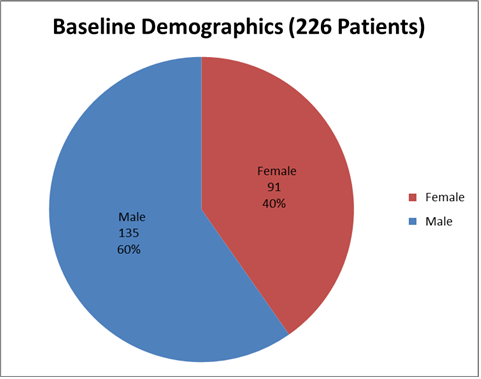
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex
Source: From Clinical Reviewer
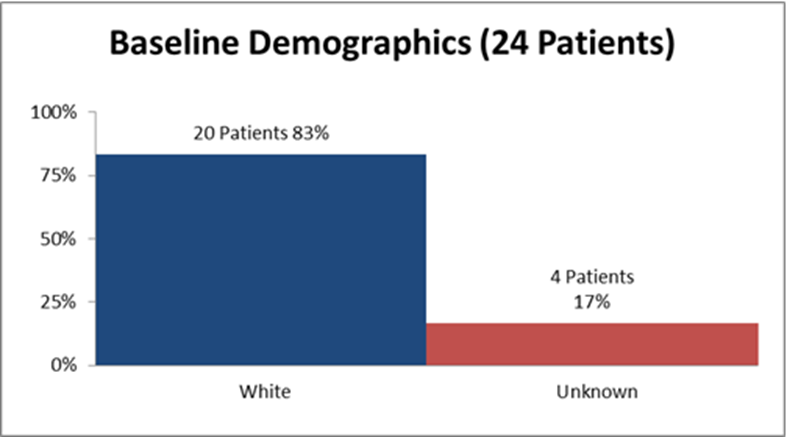
Figure 2 and Table 1 summarize how many patients by race were enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Source: From Clinical Reviewer
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage (%) |
|---|---|---|
| White | 20 | 83 |
| Unknown | 4 | 17 |
Source: From Clinical Reviewer
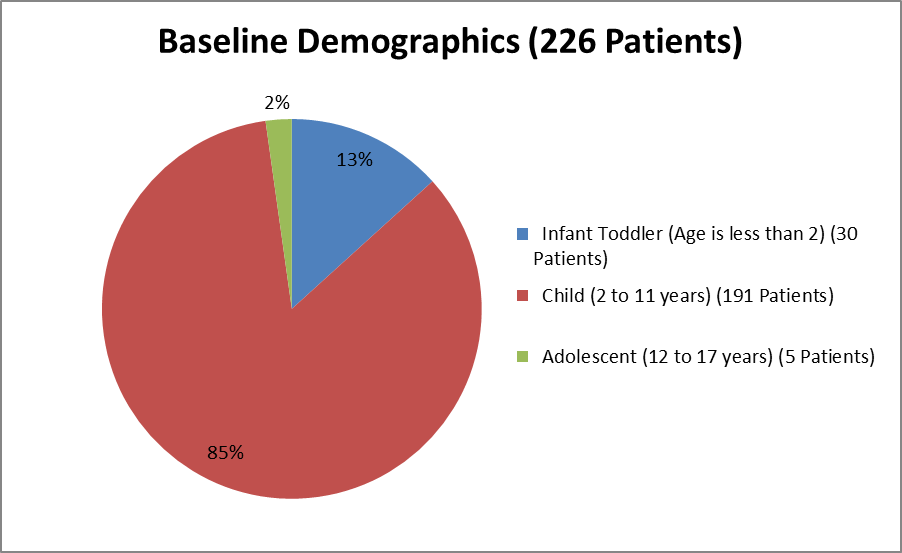
Figure 3. Baseline Demographics by Age
Source: From Clinical Reviewer
Who participated in the trials?
The table below summarizes demographic information for the trials of patients with PDs. The treatment group represented in the table includes patients for whom efficacy data was available. Baseline demographics for the safety population for patients with PDs were similar.
Table 6. Baseline Demographics, Peroxisomal Disorders (Efficacy)
| Demographic Parameter | Treatment Group N=24 |
|---|---|
| Sex | |
| Male | 16 (67) |
| Female | 8 (33) |
| Age | |
| Mean years (SD) | 1.6 (2.2) |
| Median (years) | 0.9 |
| Min, Max (years) | 0.2, 10.1 |
| Age Group | |
| <17 | 24 (100) |
| >=17 | 0 |
| >=65 years | 0 |
| >=75 years | 0 |
| Unknown | 0 |
| Race | |
| White | 20 (83) |
| Black or African American | 0 |
| Asian | 0 |
| American Indian or Alaska Native | 0 |
| Native Hawaiian or Other Pacific Islander | 0 |
| Unknown | 4 (17) |
| Ethnicity | |
| Hispanic or Latino | 2 (8) |
| Not Hispanic or Latino | 21 (88) |
| Unknown | 1 (4) |
Source: From Clinical Reviewer
How were the trials designed?
CHOLBAM was assessed in a main trial (Trial 1) that treated patients with peroxisomal disorders including Zellweger spectrum disorders. All patients in Trial 1 received CHOLBAM for 18 years. The extension trial (Trial 2) included some patients who had been treated during the main trial (Trial 1), as well as patients who were newly treated with CHOLBAM. Response to CHOLBAM was assessed using several measurements, including liver tests, body weight, and survival while being treated.
How were the trials designed?
CHOLBAM was assessed in patients with peroxisomal disorders including Zellweger spectrum disorders in two trials. Trial 1 was a non-randomized, open-label, uncontrolled trial in over an 18 year period. Trial 2 was an extension trial of new patients along with patients who rolled-over from Trial 1. There was also additional efficacy data from published case reports of three patients.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION