Drug Trials Snapshot: DALVANCE (dalbavancin)
Drug Trials Snapshot
DALVANCE (dalbavancin)
Dahl-vans
Durata Therapeutics, Inc
Approval date: May 23, 2014
DRUG TRIALS SUMMARY:
What is the drug for?
DALVANCE is used to treat serious bacterial skin infections known as acute bacterial skin and skin structure infections (ABSSSI). DALVANCE should be used only to treat infections that are proven or strongly suspected to be caused by susceptible strains of Gram-positive microorganisms.
How do I use this drug?
DALVANCE is recommended as a two-dose regimen, each dose separated by one week. The drug is administered by a health care professional as an intravenous infusion (IV) over the course of about 30 minutes.
What are the benefits of this drug?
The results of the clinical studies showed a majority of patients treated with DALVANCE stopped the spread of their skin infection and eliminated fever within 2-3 days.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: DALVANCE was shown to be similarly effective in men and women.
- Race: Subgroup analyses were conducted for non-whites and whites, but the number of patients in the non-white subgroup was limited. If there were differences in response to DALVANCE versus the comparator, it may not have been able to be detected.
- Age: DALVANCE is similarly effective in patients above and below age 65.
What are the possible side effects?
The most common adverse reactions in patients treated with DALVANCE were nausea (5.5%), headache (4.7%), and diarrhea (4.4%). Other side effects included vomiting, rash, and itching. Serious allergic reactions, reactions related to the injection of DALVANCE, and an elevation of liver enzymes can also occur.
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The frequency of side effects was similar among men and women.
- Race: Generally, non-whites had more side effects than whites in the clinical trials, whether they were treated with DALVANCE or the comparison drug (Vancomycin/linezolid).
- Age: The frequency of side effects was similar across all age groups studied.
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved DALVANCE on evidence from two clinical trials with a total of 1,312 adults who were diagnosed with a bacterial skin infection known as ABSSSI. The studies were conducted in North America, Eastern Europe, Europe, South Korea, Taiwan, South Africa, and Israel.
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex
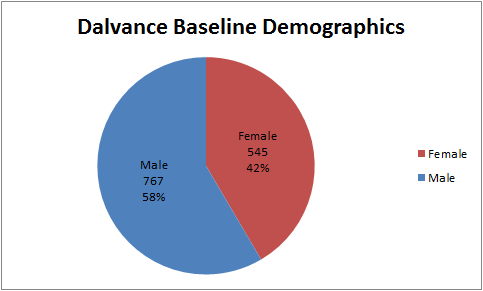
Source: Adapted from FDA Medical Review, Table 9
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trials used to evaluate the efficacy.
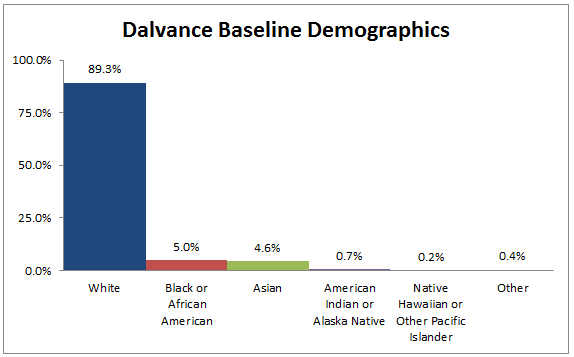
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Medical Review, Table 9
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1171 | 89.3% |
| Black or African American | 65 | 5.0% |
| Asian | 60 | 4.6% |
| American Indian or Alaska Native | 9 | 0.7% |
| Native Hawaiian or Other Pacific Islander | 2 | 0.2% |
| Other | 5 | 0.4% |
Source: Adapted from FDA Medical Review, Table 9
How was the study designed?
DALVANCE was approved by the FDA based on two, similar clinical studies with a total of 1312 patients. Assigned randomly to a treatment group, 659 patients were given DALVANCE and 653 others received a combination of Vancomycin and linezolid, which has been the standard therapy for treating ABSSSI. Neither the patients nor the health care professionals administering the drug knew which patients were taking DALVANCE and which patients were taking Vancomycin/linezolid, until after the drug trial was complete. At 48 and 72 hours, patients were evaluated to see if the infection had stopped spreading and fever was gone.
Safety evaluations of DALVANCE were based on seven trials including prior trials that were not used for the evaluation of its efficacy. Figure 3 summarizes how many men and women were enrolled in the clinical trials used to assess safety.
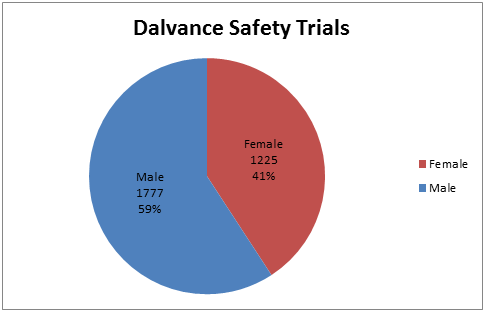
Figure 3. Demographics of Trials Used to Assess Safety by Sex
Source: Adapted from FDA Medical Review, Table 9
Figure 4 summarizes the percentage of patients by race enrolled in the clinical trials used to assess safety.
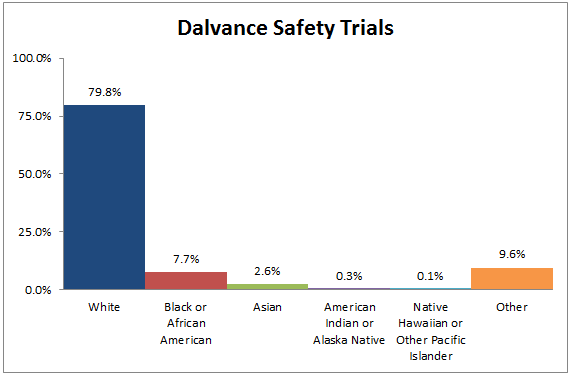
Figure 4. Demographics of Trials Used to Assess Safety by Race
Source: Adapted from FDA Medical Review, Table 9
Table 2. Demographics of Safety Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2396 | 79.8% |
| Black or African American | 231 | 7.7% |
| Asian | 77 | 2.6% |
| American Indian or Alaska Native | 9 | 0.3% |
| Native Hawaiian or Other Pacific Islander | 2 | 0.1% |
| Other | 287 | 9.6% |
What are the results of the efficacy study?
DALVANCE worked similarly to the standard therapy of Vancomycin/linezolid in stopping the spread of ABSSSI and eliminating fever within 48 to 72 hours.
What are the results of the trials used to assess safety?
The most common side effects were nausea. More serious side effects included allergic reactions, effects related to the injection of DALVANCE, and an elevation of liver enzymes.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
MEDICAL REVIEW (PDF - 5.58MB)
DRUG LABEL (PDF - 409KB)