Drug Trials Snapshot: FARYDAK (panobinostat)
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the FARYDAK Prescribing Information for complete information.
DRUG TRIALS SNAPSHOT SUMMARY:
FARYDAK (panobinostat)
(FAYR-ah-dak)
Novartis Pharmaceuticals Corporation
Approval date: February 23, 2015
What is the drug for?
Multiple myeloma is a form of blood cancer that begins in a type of white blood cell called a plasma cell. Multiple myeloma causes plasma cells to rapidly multiply and crowd out other healthy blood cells from the bone marrow. FARYDAK works by slowing the over-development of plasma cells in patients with multiple myeloma or causing the death of these dangerous cells.
FARDYAK is approved to treat people with multiple myeloma who have received at least two prior standard therapies, including bortezomib, a type of chemotherapy, and a second type of therapy called an immunomodulatory agent. FARYDAK is to be used in combination with bortezomib and dexamethasone, which is also used to kill myeloma plasma cells.
FARYDAK was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
How is the drug used?
FARYDAK is a capsule that is taken three times a week with chemotherapy.
What are the benefits of this drug?
In the main trial that supported the FDA approval of FARYDAK, half of patients treated with FARYDAK in addition to bortezomib and dexamethasone lived without their multiple myeloma getting worse for 10.6 months or longer, compared to 5.8 months for patients who received bortezomib and dexamethasone alone. It is not known whether FARYDAK will help patients live longer or feel or function better.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the trial, patients in the FARYDAK (in addition to bortezomib and dexamethasone) group lived approximately 10.6 months without their disease progressing, compared to approximately 5.8 month in the group receiving Placebo (in addition to bortezomib and dexamethasone).
Table 3 shows the time of Progression Free Survival in the FARYDAK (in addition to bortezomib and dexamethasone) arm compared to the Placebo (in addition to bortezomib and dexamethasone) arm.
Table 3. Efficacy Results from the Clinical Trial in Patients who Received Prior Treatment with Bortezomib and an Immunomodulating Agent
|
|
FARYDAK |
Placebo |
|---|---|---|
|
Progression-Free Survival |
||
|
Median, month [95% CI] |
10.6 [7.6, 13.8] |
5.8 [4.4, 7.1] |
|
Hazard ratio [95% CI]1 |
0.52 [0.36, 0.76] |
|
1 Hazard ration obtained from stratified Cox model
Source: Extracted from FARYDAK Package Insert, Table 6
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: FARYDAK was similarly effective in men and women.
- Race: FARYDAK was similarly effective in Whites and Asians. Because the number of non-White, non-Asian patients in the trial was limited, it was not possible to determine whether there were any clinically meaningful differences.
- Age: FARYDAK was similarly effective in patients above and below age 65.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The table below summarizes Progression-Free Survival for age, sex, and race groups.
Table 4. Subgroup Analyses of Progression-Free Survival for the Trial
|
|
FARYDAK+BTZ+DEX |
PBO+BTZ+DEX |
PAN+ BTZ+DEX vs. PBO+BTZ+DEX |
||
|---|---|---|---|---|---|
|
|
N=387 |
N=381 |
|||
| Subgroup |
N |
Number of events/censored patients |
N |
Number of events/censored patients |
|
|
All patients |
387 |
207/180 |
381 |
260/121 |
0.63 (0.52, 0.76) |
|
Age |
|||||
|
65 |
225 |
120/105 |
220 |
156/64 |
0.59 (0.46, 0.76) |
|
65 years |
162 |
87/75 |
161 |
104/57 |
0.72 (0.53, 0.96) |
|
Sex |
|||||
|
Male |
202 |
113/89 |
205 |
142/63 |
0.54 (0.41, 0.70) |
|
Female |
185 |
94/91 |
176 |
118/58 |
0.76 (0.57, 1.00) |
|
Race |
|||||
|
White |
249 |
139/110 |
250 |
169/81 |
0.69 (0.55, 0.86) |
|
Asian |
128 |
62/66 |
104 |
71/33 |
0.54 (0.38, 0.78) |
|
Other |
10 |
6/4 |
27 |
20/7 |
0.77 (0.27, 2.19) |
CI=confidence interval
Extracted from Statistical Review, Table 7
What are the possible side effects?
FARYDAK can cause serious side effects, including severe diarrhea, severe and life-threatening heart problems and irregular heart rhythms, bleeding, infections, and liver damage.
The most common side effects of FARYDAK were diarrhea, tiredness and weakness, nausea, swelling in the arms or legs, decreased appetite, fever, and vomiting. FARYDAK was also associated with abnormalities in laboratory tests of patients’ blood. The most common were low platelets (thrombocytopenia), low white blood cell counts (leukopenia), low levels of phosphorus (hypophosphatemia), low red blood cell counts (anemia), low potassium levels (hypokalemia), low levels of sodium (hyponatremia), and increased creatinine.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial. The patient population includes all patients who were studied in the trial.
Table 5. Adverse Reactions (≥10% Incidence and ≥5% greater Incidence in FARYDAK Arm) in the Clinical Trial
| Preferred term |
FARYDAK, BTZ, Dex |
Placebo, BTZ, Dex |
|---|---|---|
|
Diarrhea |
68 |
42 |
|
Fatigue 1 |
60 |
42 |
|
Nausea |
36 |
21 |
|
Peripheral edema |
29 |
19 |
|
Decreased appetite |
28 |
12 |
|
Vomiting |
26 |
13 |
|
Pyrexia |
26 |
15 |
|
Arrhythmia2 |
12 |
5 |
|
Weight decreased |
12 |
5 |
BTZ = bortezomib; Dex = dexamethasone
1 Fatigue includes the terms: fatigue, malaise, asthenia, and lethargy
2 Arrhythmia includes the terms: arrhythmia, arrhythmia supraventricular, atrial fibrillation, atrial flutter, atrial tachycardia, bradycardia, cardiac arrest, cardio-respiratory arrest, sinus bradycardia, sinus tachycardia, supraventricular extra-systoles, tachycardia, ventricular arrhythmia, and ventricular tachycardia
Extracted from FARYDAK Package Insert, Section 6, Table 4
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of overall side effects was similar in males and females.
- Race: The risk of overall side effects was similar in Whites and Asians. Asians had a higher frequency of certain side effects (listed in MORE INFO) compared to Whites. Because the number of non-White, non-Asian patients in the trial was limited, it was not possible to determine whether there were any clinically meaningful differences.
- Age: The risk of overall side effects was similar in patients above and below age 65 years. In patients above 65 years, there were more deaths compared to patients below the age of 65. The risk of certain side effects (listed in MORE INFO) was increased in patients above the age of 65.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes all adverse events by subgroup.
Table 6. Total Adverse Events by subgroup
|
Subgroup |
FARYDAK+BTZ+DEX |
Placebo+BTZ+DEX |
|---|---|---|
|
Sex |
||
|
Male |
201/201 (100%) |
204/205 (99.5%) |
|
Female |
179/180 (99.4%) |
172/172 (100%) |
|
Race |
||
|
White |
243/244 (99.6%) |
246 (99.6 %) |
|
Asian |
127/127 (100%) |
103/103 (100%) |
|
Other |
10/10 (100%) |
27/27 (100%) |
|
Age |
||
|
65 |
221/221 (100%) |
216/217 (99.5%) |
|
65 years |
159/160 (99.4%) |
160/160 (100%) |
Source: Extracted from Company’s submission, Table 14.3.1-1.2
BTZ=bortezomib; DEX=dexamethasone
The table below summarizes selected adverse events in the clinical trials for Asians compared to Whites.
Table 7. Comparison of Selected Adverse events in Asians compared to Whites in Clinical Trial
|
Adverse Event |
Asians |
Whites |
|---|---|---|
| Diarrhea | 72 | 67 |
| Decreased appetite | 44 | 21 |
| Vomiting | 36 | 20 |
| Peripheral neuropathy | 34 | 27 |
| Constipation | 30 | 25 |
| Cough | 30 | 18 |
Source: Extracted from Clinical Review, Table 36
BTZ=bortezomib; DEX=dexamethasone
The table below summarizes the most common adverse reactions in FARYDAK-treated patients by age group.
Table 8. Most common adverse reactions in FARYDAK-treated patients >20% of patients age >65 years compared to <65 years="" in="" the="" clinical="">
|
Adverse Event |
Age <65> |
Age >65 years |
|---|---|---|
| Diarrhea | 63 | 76 |
| Fatigue | 37 | 47 |
| Nausea | 39 | 33 |
| Peripheral edema | 27 | 32 |
| Vomiting | 23 | 29 |
| Decreased appetite | 29 | 29 |
| Pyrexia | 25 | 26 |
| Constipation | 28 | 26 |
| Asthenia | 21 | 24 |
| Peripheral neuropathy | 37 | 22 |
Source: Extracted from Clinical Review, Table 35
There were 14 patients (9%) 65 years of age or older compared to 12 patients (5%) below 65 years of age who died due to a reason other than disease progression in the panobinostat arm (from Clinical Review).
WHO WAS IN THE TRIALS?
Who participated in the clinical trials?
The FDA approved FARYDAK based on evidence from a clinical trial of 768 patients with multiple myeloma that returned after previous treatment. The safety of FARYDAK was evaluated using information from 758 patients; FARYDAK was evaluated to see how well it worked by using a subset of 193 patients who had received prior treatment with two other drugs.
The trial was conducted at 194 sites in many countries, including those in North America, South America, Europe, Australia, and Asia.
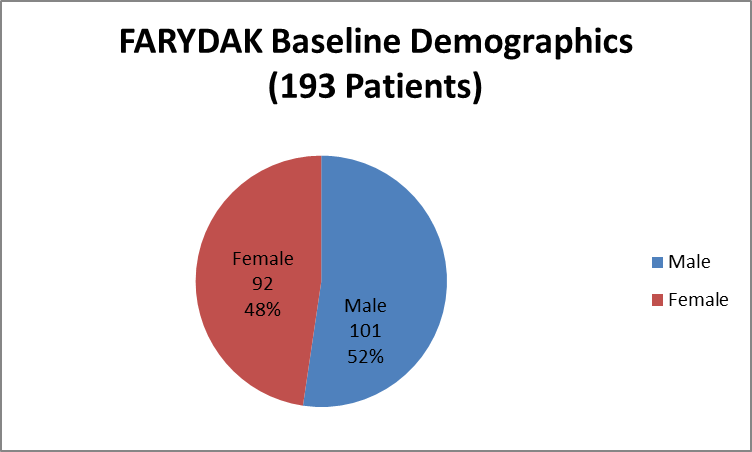
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex (Efficacy)
Source: Extracted from Clinical Review Addendum, Table 1
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials used to evaluate efficacy.
Figure 2. Baseline Demographics by Race (Efficacy)

Table 1. Baseline Demographics by Race (Efficacy)
|
Race |
Panobinostat + BD |
Placebo + BD |
|---|---|---|
|
White |
59 (62.8%) |
63 (63.6%) |
|
Asian |
34 (36.2%) |
29 (29.3%) |
|
Black or African American |
1 (1.1%) |
5 (5.1%) |
|
Other |
0 |
2 (2%) |
Extracted from: Clinical Stats Review Addendum, Table 1
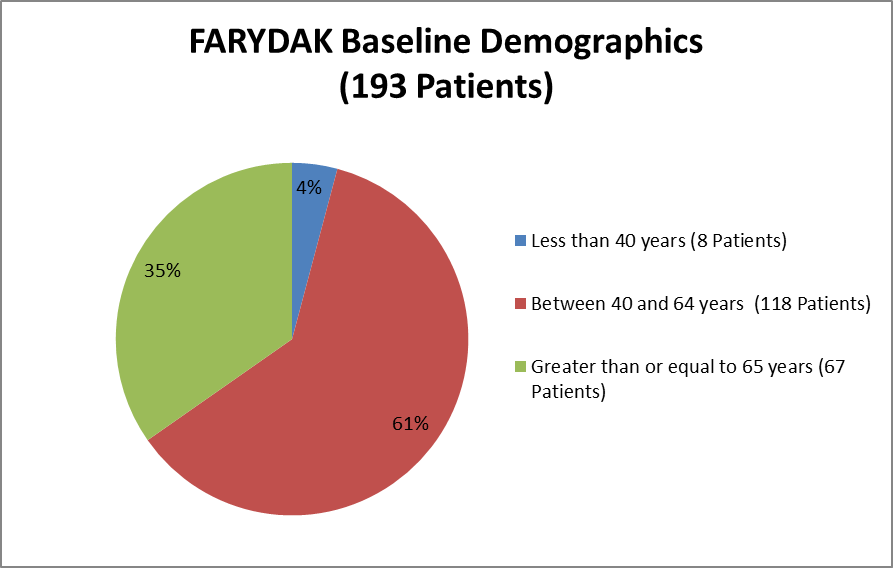
Figure 3 summarizes the percentage of patients by age group enrolled in the clinical trial for efficacy.
Figure 3. Baseline Demographics by Age (Efficacy)
Source: Extracted from Clinical Review Addendum, Table 1
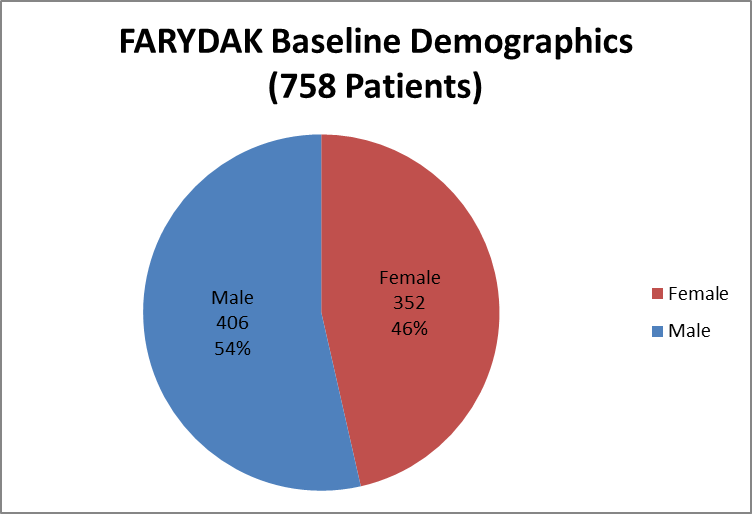
Figure 4 summarizes how many men and women were enrolled in the clinical trials used to evaluate safety.
Figure 4. Baseline Demographics by Sex (Safety)
Source: Extracted from Clinical Review, Table 11
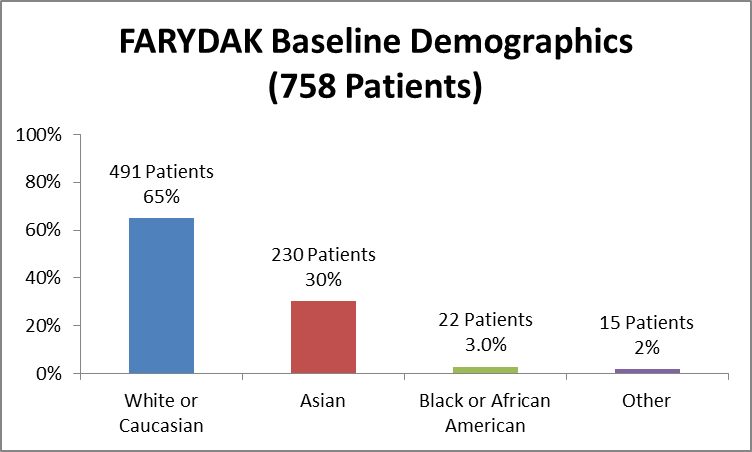
Figure 5 and Table 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate safety.
Figure 5. Baseline Demographics by Race (Safety)
Extracted from: Clinical Review, Table 11
Table 2. Baseline Demographics by Race (Safety)
|
Race |
Panobinostat + BD |
Placebo + BD |
|---|---|---|
|
White |
246 (63.7%) |
245 (65.9%) |
|
Asian |
129 (33.4%) |
101 (27.2%) |
|
Black or African American |
5 (1.3%) |
17 (4.6%) |
|
Other |
6 (1.6 %) |
9 (2.4%) |
Source: Extracted from Clinical Review, Table 11
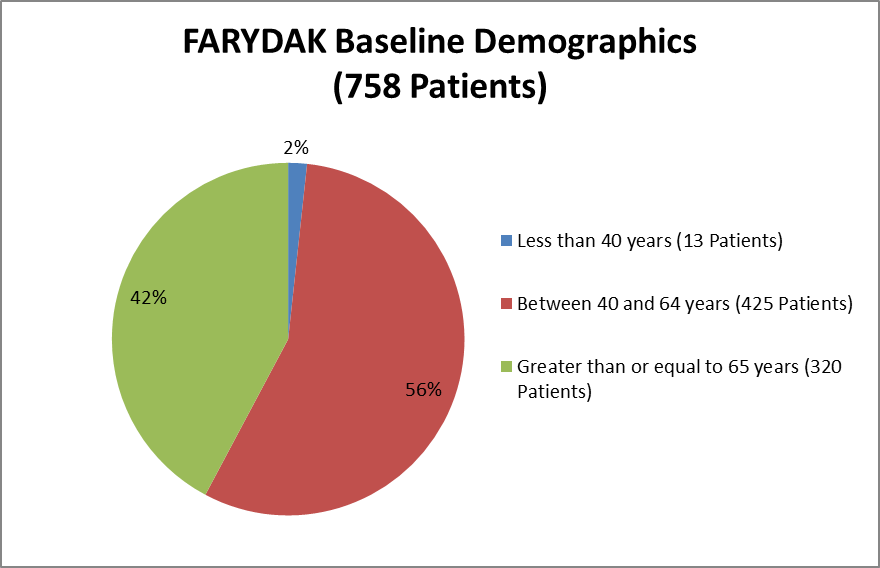
Figure 6 summarizes the percentage of patient by age group enrolled in the clinical trial for safety.
Figure 6. Baseline Demographics by Age (Safety)
Source: Extracted from Clinical Review, Table 11
Who participated in the trials?
FARYDAK was evaluated in a trial of 768 patients with relapsed multiple myeloma. Efficacy analyses were performed in a subpopulation of 193 patients who had received prior treatment with bortezomib and with lenalidomide or thalidomide. Safety analyses were performed on 758 patients: 386 who received at least one dose of panobinostat and 372 who received placebo. The demographic characteristics for both populations are summarized below.
Table 9: Demographic and Baseline Characteristics (Efficacy Analysis)
|
Panobinostat + BD |
Placebo + BD |
|
|---|---|---|
|
Age, years |
||
|
Mean (SD) |
59 (10) |
61 (9) |
|
Median |
60 |
61 |
|
Range |
28-79 |
32-77 |
|
Age Groups |
||
|
<40 |
5 (5.3%) |
3 (3.0%) |
|
40-64 |
60 (63.8%) |
58 (58.6%) |
|
≥ 65 |
29 (30.9%) |
38 (38.4%) |
|
Sex |
||
|
Male |
52 (55.3%) |
79 (49.5%) |
|
Female |
42 ( 44.7%) |
50 (50.5%) |
|
Race |
||
|
White or Caucasian |
59 (62.8%) |
63 (63.6%) |
|
Asian |
34 (36.2%) |
29 (29.3%) |
|
Black or African American |
1 (1.1%) |
5 (5.1%) |
|
Other |
0 |
2 (2.0%) |
|
Region |
||
|
Americas |
16 (17%) |
20 (20.2%) |
|
Europe |
41 (43.6%) |
49 (49.5%) |
|
Western Pacific |
36 (38.3%) |
30 (30.3%) |
|
Other |
1 (1.1%) |
0 |
Source: Extracted from Clinical Review Addendum, Table 1
Table 10: Demographic and Baseline Characteristics (Safety Analysis)
|
Panobinostat + BD |
Placebo + BD |
|
|---|---|---|
|
Age, years |
||
|
Mean (SD) |
62 (9.4) |
62 (9.3) |
|
Median |
63 |
63 |
|
Range |
28-84 |
32-83 |
|
Age Groups |
||
|
<40 |
6 (2%) |
7 (2%) |
|
40-64 |
218 (56%) |
207 (56%) |
|
≥ 65 |
162 (42%) |
158 (42%) |
|
Sex |
||
|
Male |
206 (53%) |
200 (54%) |
|
Female |
180 (47%) |
172 (46%) |
|
Race |
||
|
White or Caucasian |
246 (64%) |
245 (66%) |
|
Asian |
129 (33%) |
101 (27%) |
|
Black or African American |
5 (1%) |
17 (5%) |
|
Other |
6 (2%) |
9 (2%) |
|
Region |
||
|
Americas |
48 (12.4%) |
72 (19.4%) |
|
Europe |
195 (50.5%) |
178 (47.8%) |
|
Western Pacific |
133 (34.5%) |
105 (28.2%) |
|
Other |
10 (2.6%) |
17 (4.6%) |
Source: Extracted from Clinical Review, Table 11 and Statistical Review, Table 7
HOW WERE THE TRIALS DESIGNED?
The trial randomly assigned half the patients to take a combination of FARYDAK in addition to bortezomib and dexamethasone and the other half a placebo in addition to bortezomib and dexamethasone. Neither the patients nor the healthcare providers knew which patients were receiving which treatment. The treatment continued until the disease progressed, the side effects became too toxic, or the patient decided to discontinue the study.
How were the trials designed?
The main trial was a large, international, randomized (1:1), double-blinded, placebo-controlled trial in which 768 subjects with relapsed multiple myeloma were treated with bortezomib and dexamethasone with or without FARYDAK. Patients with 1 to 3 prior treatments were eligible. The primary efficacy endpoint was Investigator-assessed progression-free survival (PFS). Randomization was stratified by the number of prior lines of therapy and by prior use of bortezomib. Patients were to be treated for a maximum of 48 weeks in two 24-week phases.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
Prescribing Information
MEDICAL REVIEW