Drug Trials Snapshot: Ga 68 PSMA-11
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the Ga 68 PSMA-11 Prescribing Information for complete information.
Ga 68 PSMA-11
UCLA Nuclear Medicine, UCSF Nuclear Medicine
Approval date: December 1, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
Ga 68 PSMA-11 is a drug used for detection of specific cancer lesions in men with prostate cancer
- whose newly diagnosed cancer could be cured with the initial treatment, or
- who have been treated for prostate cancer but have high prostate-specific antigen (PSA) in their blood. High PSA in the blood of these patients is a suspicious sign that cancer is coming back or spreading.
How is this drug used?
Ga 68 PSMA-11 is an injection given by a health care provider in the vein (intravenous) in preparation for an imaging test that can help detect cancer (called positron emission tomography or PET scan imaging).
What are the benefits of this drug?
PET imaging done after Ga 68 PSMA-11 injection showed sites of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer.
What are the benefits of this drug?
The table below summarizes efficacy results for Trial 1 based on comparison of the majority PET reads to pelvic lymph node histopathology results at the patient-level with region matching, such that at least one true positive region defines a true positive patient.
Table 1. Patient-Level Performance of Ga 68 PSMA-11 PET for Detection of Pelvic Lymph Node Metastasis* in Trial 1 (n=123)
| Histopathology | Predictive value** (95% CI) | |||
|---|---|---|---|---|
| Positive | Negative | |||
| PET scan |
Positive | 14 | 9 | PPV 61% (41%, 81%) |
| Negative | 16 | 84 | NPV 84% (79%, 91%) |
|
| Total | 30 | 93 | ||
| Diagnostic performance (95% CI) | Sensitivity 47% (29%, 65%) |
Specificity 90% (84%, 96%) |
||
*with region matching where at least one true positive region defines a true positive patient
**PPV: positive predictive value, NPV: negative predictive value
Ga 68 PSMA-11 Prescribing Information
In Trial 2, 192 patients (91%) out of 210 evaluable patients were found to be true positive in one or more regions based on a composite reference standard (95% confidence interval: 88%, 95%). The table below summarizes how often the PET scan was positive in patients with biochemical evidence of recurrent prostate cancer based on their PSA level.
Table 2. Patient-Level Ga 68 PSMA-11 PET Results and Percent PET Positivity Stratified by Serum PSA Level in Trial 2 (n=628)*
| PSA (ng/mL) |
PET positive patients | PET negative patients | Percent PET positivity*** (95% CI) | |||
|---|---|---|---|---|---|---|
| Total | TP** | FP** | Without reference standard | |||
| With reference standard | ||||||
| <0.5 | 48 | 11 | 1 | 36 | 87 | 36% (27%, 44%) |
| 12 | ||||||
| ≥0.5 and <1 | 44 | 15 | 3 | 26 | 35 | 56% (45%, 67%) |
| 18 | ||||||
| ≥1 and <2 | 71 | 29 | 1 | 41 | 15 | 83% (75%, 91%) |
| 30 | ||||||
| ≥2 | 299 | 137 | 13 | 149 | 29 | 91% (88%, 94%) |
| 150 | ||||||
| Total | 462 | 192 | 18 | 252 | 166 | 74% (70%, 77%) |
| 210 | ||||||
*7 patients were excluded from this table due to protocol deviations
**TP: true positive, FP: false positive
***Percent PET positivity = PET positive patients/total patients scanned/p>
Ga 68 PSMA-11 Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All trial participants were men; therefore, sex differences cannot be determined.
- Race: Majority of participants in the clinical trials were White. Differences among races could not be determined because of the small number of participants from other races.
- Age: Ga 68 PSMA-11 worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by age.
Table 3. Efficacy Subgroup Analysis by Age in Trial 1
| Age | Efficacy endpoint | Point estimate (95% confidence interval) |
|---|---|---|
| < 65 years | Sensitivity | 6/16 = 0.38 (0.14, 0.61) |
| Specificity | 32/36 = 0.89 (0.76, 0.97) |
|
| PPV | 6/10 = 0.60 (0.26, 0.88) |
|
| ≥ 65 years | Sensitivity | 8/14 = 0.57 (0.31, 0.83) |
| Specificity | 52/57 = 0.91 (0.84, 0.99) |
|
| PPV | 8/13 = 0.62 (0.35, 0.88) |
PPV=positive predictive value
FDA Review
Table 3. Efficacy Subgroup Analysis by Age in Trial 2.
| Age | Efficacy endpoint | Point estimate (95% confidence interval) |
|---|---|---|
| < 65 years | Patient-level PPV | 43/45 = 0.96 (0.85, 0.99) |
| Region-level PPV | 51/53 = 0.96 (0.87, 0.99) |
|
| ≥ 65 years | Patient-level PPV | 157/172 = 0.91 (0.86, 0.95) |
| Region-level PPV | 178/196 = 0.91 (0.86, 0.94) |
PPV=positive predictive value
Table includes only those patients with available imaging and reference standards, which is 46%.
FDA Review
What are the possible side effects?
Ga 68 PSMA-11 is a radioactive drug which will increase lifetime radiation exposure.
The most common side effects of Ga 68 PSMA-11 are nausea, diarrhea and dizziness.
What are the possible side effects?
The safety of Ga 68 PSMA-11 Injection was evaluated in 960 patients, each receiving one dose of Ga 68 PSMA-11 Injection. The most commonly reported adverse reactions were nausea, diarrhea, and dizziness, occurring at a rate of < 1%.
Ga 68 PSMA-11 Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: All trial participants were men, therefore sex differences in side effects could not be determined.
- Race: Majority of participants in the clinical trials were White. Differences in side effects among races could not be determined due to small number of participants from other races.
- Age: The occurrence of side effects between patients younger and older than 65 years of age was similar.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The analysis of side effects among age groups is presented below.
Table 4. Occurrence of Adverse Events per Age Group
| AGE GROUP | ||
|---|---|---|
| <65 years (N=273) |
≥65 years (N=687) |
|
| Patients with any Adverse Event | 7 (3%) | 21 (3%) |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved Ga 68 PSMA-11 based on evidence from two clinical trials (Trial 1/NCT0336847 identical to NCT02919111 and Trial 2/NCT02940262 identical to NCT02918357) of male patients with prostate cancer. Some patients were recently diagnosed with the prostate cancer. Other patients were treated before, but there was suspicion that the cancer was spreading because of rising prostate specific antigen or PSA. The trials were conducted at 2 sites in the USA.
The figure below summarizes how many male participants were in the clinical trials.
Figure 1. Demographics by Sex
FDA Review
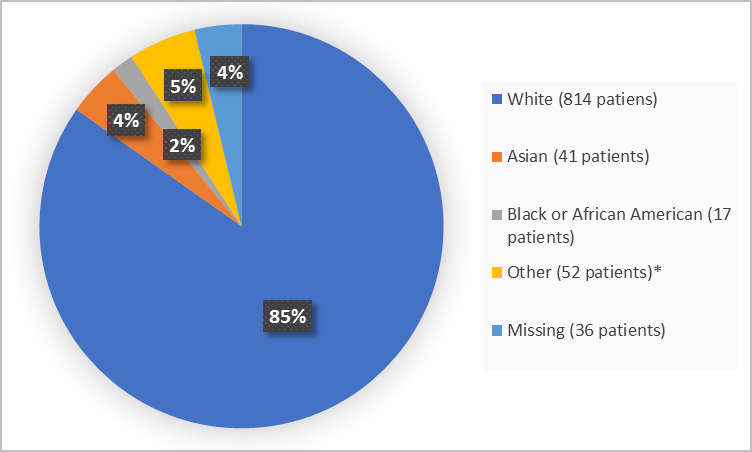
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race
*Includes American Indian or Alaska Native (2), Native Hawaiian or Pacific Islander (5) and Other (45).
FDA Review
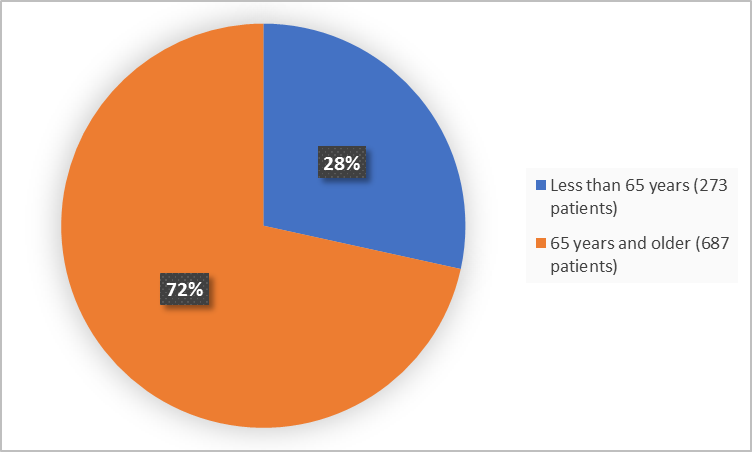
Figure 3 summarizes the percentage of patients by age group in the clinical trials.
Figure 3. Demographics by Age
FDA Review
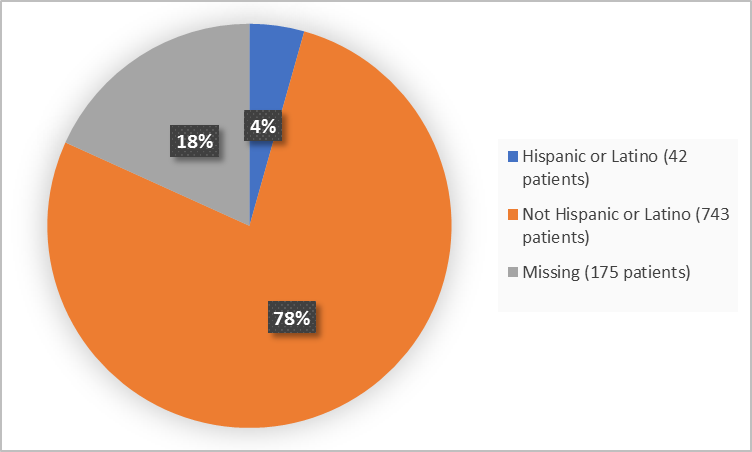
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity
FDA Review
Who participated in the trials?
The table below summarizes demographics of patients in both trials (safety population).
Table 5. Demographics of Patients in the Clinical Trials (Safety Population)
| Demographic Parameters | Trial 1 (N=325) n (%) |
Trial 2 (N=635) n (%) |
TOTAL (N=960) n (%) |
|---|---|---|---|
| Sex | |||
| Men | 325 (100) | 635 (100) | 960 (100) |
| Age | |||
| Median (years) | 69 | 69 | 69 |
| Min, max (years) | 40,93 | 44,95 | 40, 93 |
| Age Group | |||
| < 65 years | 101 (31) | 172 (27) | 273 (28) |
| 65 years and older | 224 (69) | 463 (73) | 687 (72) |
| Race | |||
| White | 269 (83) | 545 (86) | 814 (85) |
| Black or African American | 6 (2) | 11 (2) | 17 (2) |
| Asian | 18 (6) | 23 (4) | 41 (4) |
| American Indian or Alaska Native | 1 (<1) | 1 (<1) | 2 (<1) |
| Native Hawaiian or Pacific Islander | 4 (1) | 1 (<1) | 5 (0.5) |
| Other | 20 (6) | 25 (4) | 45 (5) |
| Missing | 7 (2) | 29 (5) | 36 (4) |
| Ethnicity | |||
| Hispanic or Latino | 12 (4) | 30 (5) | 42 (4) |
| Not Hispanic or Latino | 297 (91) | 446 (70) | 743 (77) |
| Missing | 16 (5) | 159 (25) | 175 (18) |
| Region | |||
| United States | 325 (100) | 635 (100) | 960 (100) |
FDA Review
How were the trials designed?
There were two trials that evaluated benefits and side effects of Ga 68 PSMA-11.
Trial 1 enrolled patients who were recently diagnosed with prostate cancer and were awaiting surgery for the removal of the prostate and the nearby lymph nodes. Trial 2 enrolled patients who were already treated for prostate cancer but had rising PSA levels, suspicious for cancer spreading.
The benefit of Ga 68 PSMA-11 in Trial 1 was evaluated by measuring the successful detection of cancer lesions using Ga 68 PSMA-11 PET/CT imaging in comparison to post-surgery lymph tissue results (histopathology of the lymph nodes).
The benefit of Ga 68 PSMA-11 in Trial 2 was evaluated by comparing the agreement in cancer lesion detection between images done with Ga 68 PSMA-11 and one of the standard tests for detecting cancer spreading.
How were the trials designed?
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective, open-label trials (Trial 1 and Trial 2) in men with prostate cancer.
Trial 1 enrolled patients with biopsy-proven prostate cancer who were considered candidates for prostatectomy and pelvic lymph node dissection. Patients underwent Ga 68 PSMA-11 PET/CT imaging followed by the surgery. The images were read by three blinded independent readers and compared to histopathology obtained from dissected pelvic lymph nodes.
Trial 2 enrolled patients with biochemical evidence of recurrent prostate cancer after definitive therapy defined by serum PSA of >0.2 ng/mL more than 6 weeks after prostatectomy or by an increase in serum PSA of at least 2 ng/mL above nadir after definitive radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT or PET/MR from mid-thigh to skull base. The images were read by three blinded independent readers and compared to at least one of the following: histopathology, imaging (bone scintigraphy, CT, or MRI) acquired at baseline or within 12 months after Ga 68 PSMA-11 PET, or serial serum PSA.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION