Drug Trials Snapshot: KLISYRI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the KLISYRI Package Insert for complete information.
KLISYRI (tirbanibulin)
(klye si' ree )
Almirall, LLC
Approval date: December 14, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KLISYRI is used on the skin to treat patients with actinic keratosis on the face or scalp.
Actinic keratosis is a common skin disease caused by long-term exposure to the sun and/or indoor tanning. If left untreated, AK may progress to skin cancer.
How is this drug used?
KLISYRI is an ointment. One package of ointment is applied on the affected area of face or scalp 1 time a day for 5 days in a row.
What are the benefits of this drug?
More patients achieved complete clearance of AK after 5 days of treatment with KLISYRI in comparison to those who were treated with control ointment (vehicle).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients in Trials 1 and 2. The primary efficacy endpoint was complete (100%) clearance of AK lesions in the treatment area, defined as the proportion of subjects at Day 57 with no clinically visible AK lesions in the treatment area.
Table 1. Complete (100%) AK Clearance Rates on Day 57 for the Two Phase 3 Studies (Intent to Treat [ITT] Population
|
|
Trial 1 |
Trial 2 |
||||||
|---|---|---|---|---|---|---|---|---|
|
|
KLISYRI |
Vehicle |
Treatment difference |
95% Confidence Interval |
KLISYRI |
Vehicle |
Treatment difference |
95% Confidence Interval |
|
All patients |
77/175 (44%) |
8/176 (5%) |
40%a |
(31.6%, 47.5%)a |
97/178 (54%) |
22/173 (13%) |
42%a |
(33.1%, 50.7%)a |
|
Face |
60/119 (50%) |
7/121 (6%) |
45% |
-- |
73/119 (61%) |
16/118 (14%) |
48% |
-- |
|
Scalp |
17/56 (30%) |
1/55 (2%) |
29% |
-- |
24/59 (41%) |
6/55 (11%) |
30% |
-- |
a Based on Mantel-Haenszel method
KLISYRI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
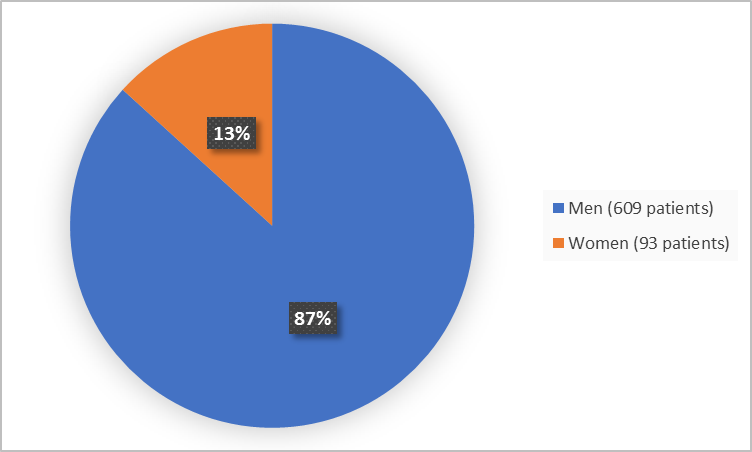
- Sex: Almost all patients in the trials were men, therefore differences in how KLISYRI worked between men and women could not be determined.
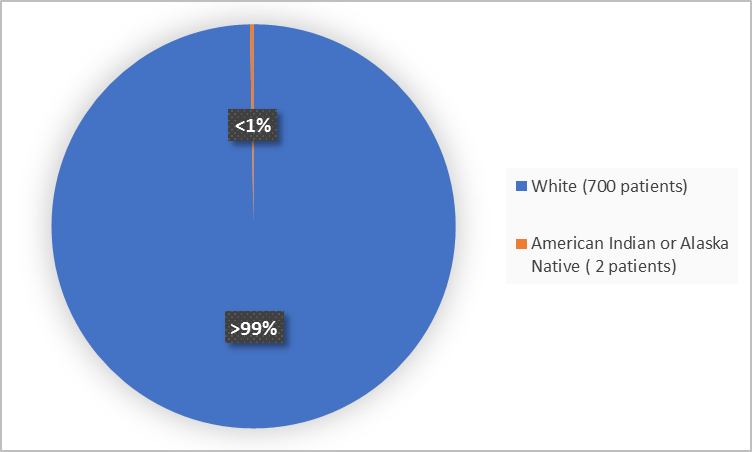
- Race: Almost all patients in the trials were White therefore, differences in how KLISYRI worked among races could not be determined.
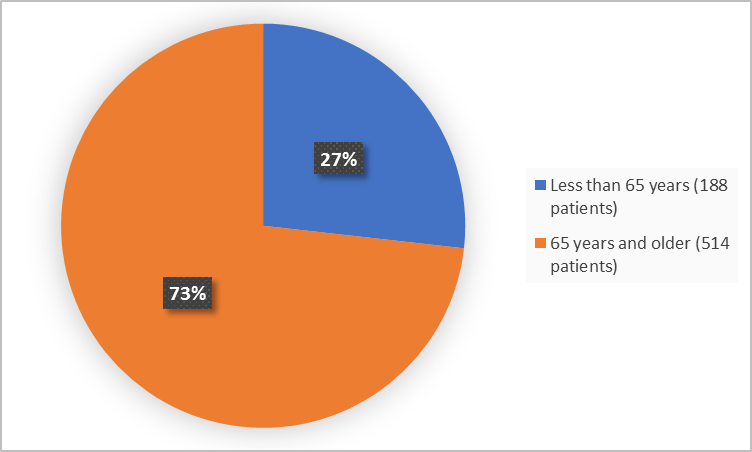
- Age: KLISYRI worked similarly between patients younger than and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex, and age in individual trials
Table 2. Complete (100%) Clearance Rates at Day 57 in for Subgroups Based on Sex and Age for Trial 1
|
|
Trial 1 |
||
|---|---|---|---|
|
KLISYRI |
Vehicle |
Difference and 95% CI |
|
|
Sex |
|||
|
Women, n/N (%) |
17/28 (60.7%) |
3/22 (13.6%) |
47.1% (20.1%,68.9%) |
|
Men, n/N (%) |
60/147 (40.8%) |
5/154 (3.3%) |
37.6% (26.7%,47.8%) |
|
Age |
|||
|
<65 years, n/N (%) |
23/51 (45.1%) |
1/42 (2.4%) |
42.7% (23.0%, 59.9%) |
|
≥ 65 years, n/N (%) |
54/124 (43.6%) |
7/134 (5.2%) |
38.3% (26.6%,49.2%) |
Table 3. Complete (100%) Clearance Rates at Day 57 in for Subgroups Based on Sex and Age for Trial 2
|
|
Trial 2 |
||
|---|---|---|---|
|
KLISYRI |
Vehicle |
Difference and 95% CI |
|
|
Sex |
|||
|
Women, n/N (%) |
17/20 (85.0%) |
3/23 (13.0%) |
72.0% (45.2%,88.9%) |
|
Men, n/N (%) |
80/158 (50.6%) |
19/150 (12.7%) |
38.0% (27.2%,48.0%) |
|
Age |
|||
|
<65 years, n/N (%) |
35/56 (62.5%) |
4/39 (10.3%) |
52.2% (33.4%,68.1%) |
|
≥ 65 years, n/N (%) |
62/122 (50.8%) |
18/134 (13.4%) |
37.4% (25.6%,48.3%) |
Note: Complete (100%) clearance rate was defined as the proportion of subjects with no clinically visible AK lesions in the treatment area at Day 57.
1 Based on Clopper-Pearson Exact 95% CI. Note that for subgroup face and scalp, the reported 95% CI is for exploratory rather than confirmatory purpose
Adapted from FDA Review
What are the possible side effects?
KLISYRI may cause eye irritation.
The most common side effect of KLISYRI are local skin reactions, application site pruritus, and application site pain.
What are the possible side effects (results of trials used to assess safety)?
Presented below are common adverse reactions in the pooled trials.
Table 4. Adverse Reactions Occurring in ≥2% of Subjects in 2 Controlled Clinical Trials– Pooled Safety Population
|
Adverse Reaction |
KLISYRI |
Vehicle |
|---|---|---|
|
Number of Patients (%) with any adverse reaction (possibly related to treatment) |
56 (16%) |
35 (10%) |
|
Application site pruritus |
32 (9%) |
21 (6%) |
|
Application site paina |
35 (10%) |
11 (3%) |
a Application site pain includes pain, tenderness, stinging, and burning sensation at the application site.
KLISYRI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Almost all patients in the trials were men, therefore differences in the occurrence of side effects between men and women could not be determined.
- Race: Almost all patients in the trials were White, therefore differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar between patients younger than and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of the most common adverse reactions, by sex and age subgroups.
Table 5. Subgroup Analysis of Common Adverse Reactions by Sex
|
|
KLISYRI (N=175) |
Vehicle (N=176) |
||
|---|---|---|---|---|
|
Women |
Men |
Women |
Men |
|
|
Application Site Pruritis |
6/48 (13) |
26/305 (9) |
1/45 (2) |
20/304 (7) |
|
Application Site Pain |
5/48 (10) |
30/305 (10) |
0/45 (0) |
11/304 (4) |
Table 6. Subgroup Analysis of Common Adverse Reactions by Age
|
|
KLISYRI (N=175) |
Vehicle (N=176) |
||
|---|---|---|---|---|
|
<65 years |
≥ 65 years |
<65 years |
≥ 65 years |
|
|
Application Site Pruritis |
10/107 (9) |
22/246 (9) |
4/81 (5) |
17/268 (6) |
|
Application Site Pain |
12/107 (11) |
23/246 (9) |
3/81 (4) |
8/268 (3) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved KLISYRI based on evidence from two clinical trials (Trial 1/ NCT0328547 and Trial 2/03285490) of 702 adult patients with actinic keratosis on the face or scalp. The trials were conducted at 62 sites in the United States.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Demographics by Age
FDA Review
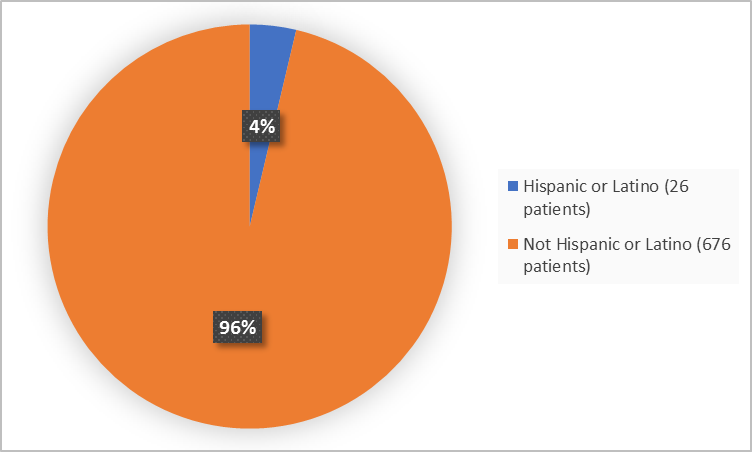
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity
FDA Review
Who participated in the trials?
The table below summarizes demographics of the population in the trials.
Table 7. Baseline Demographics
|
|
Trial 1 |
Trial 2 |
Total |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Men |
301 (86) |
308 (88) |
609 (87) |
|
Women |
50 (14) |
43 (12) |
93 (13) |
|
Race, n (%) |
|||
|
White |
350 (>99) |
350 (>99) |
700 (>99) |
|
American Indian or Alaska Native |
1 (<1) |
1 (<1) |
2 (<1) |
|
Age (years) |
|||
|
Median |
70.0 |
70.0 |
70.0 |
|
Min, max |
45, 97 |
47, 93 |
45, 97 |
|
Age group (years), n (%) |
|||
|
<65 |
93 (26) |
95 (27) |
188 (27) |
|
≥65 |
258 (74) |
256 (73) |
514 (73) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
7 (2) |
19 (5) |
26 (4) |
|
Not Hispanic or Latino |
344 (98%) |
332 (95) |
676 (96) |
|
Region |
|||
|
USA |
351 (100) |
351 (100) |
702 (100) |
FDA Review
How were the trials designed?
The benefit and side effects of KLISYRI were evaluated in two clinical trials which enrolled adult patients with actinic keratosis on the face or scalp.
Patients received once daily treatment with either KLISYRI or inactive control ointment for 5 consecutive days to the single predetermined area where they had actinic keratosis. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The benefit of KLISYRI in comparison to control was assessed after 57 days by comparing the percentage of patients who did not have any AK on the treatment area (100% clearance).
How were the trials designed?
The safety and efficacy of KLISYRI were established in two double-blind, vehicle-controlled clinical trials that enrolled adult patients with actinic keratosis on the face or scalp. Patients had 4 to 8 clinically typical, visible, and discrete AK lesions in a contiguous area of 25 cm2 on the face or scalp.
Patients received 5 consecutive days of once daily treatment with either KLISYRI or vehicle control to the treatment field.
The primary efficacy endpoint was complete (100%) clearance of AK lesions in the treatment area, defined as the proportion of patients at Day 57 with no clinically visible AK lesions in the treatment area.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.