Drug Trials Snapshot: LENVIMA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the LENVIMA Package Insert for complete information.
LENVIMA™ (lenvatinib)
lehn-veema
Eisai Inc.
Approval date: February 13, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LENVIMA is a medicine used to treat people with a type of thyroid cancer called differentiated thyroid cancer (DTC) that is progressing and can no longer be treated with a therapy called radioactive iodine. LENVIMA is a type of drug called a kinase inhibitor that works by blocking certain proteins from helping cancer cells grow and divide.
How is this drug used?
LENVIMA is a capsule that is taken by mouth once daily.
What are the benefits of this drug?
In the main trial that supported the FDA approval of LENVIMA, half of the patients treated with LENVIMA were alive without tumor growth for 18.3 months or longer, compared to 3.6 months for patients who received a placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the trial, half of the patients in the LENVIMA arm were alive with no growth of their tumor for 18.3 months or longer, compared to 3.6 months for patients who received a placebo.
Table 3 summarizes information on Progression Free Survival (time from the start of treatment until tumor growth or death) in the LENVIMA arm compared to placebo.
Table 3. Efficacy Results from the Clinical Trial
| LENVIMA | Placebo |
|---|---|---|
Progression-free Survival | ||
Number of events (%) | 107 (41) | 113 (86) |
Progressive disease | 93 (36) | 109 (83) |
Death | 14 (5) | 4 (3) |
Median PFS in months (95% CI) | 18.3 (15.1, NE) | 3.6 (2.2, 3.7) |
Hazard ratio (95% CI) | 0.21 (0.16, 0.28) | |
P-value | ||
Extracted from LENVIMA Package Insert, Table 4
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: LENVIMA appeared to work similarly in men and women.
- Race: Subgroup analysis was conducted for whites and Asians. LENVIMA appeared to work similarly in these groups. The number of non-white, non-Asian patients was limited, and therefore differences in response in these groups could not be determined.
- Age: Subgroup analysis was conducted for patients below and above 65 years of age. LENVIMA appeared to work similarly in these age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Subgroup analyses were conducted for sex, race, and age.
- Sex: LENVIMA appeared to work similarly in men and women.
- Race: Subgroup analysis was conducted for whites and Asians. LENVIMA appeared to work similarly in these groups. The number of non-white, non-Asian patients was limited.
- Age: Subgroup analysis was conducted for patients below and above 65 years of age. LENVIMA appeared to work similarly in these age groups.
Figure 4. Progression Free Survival by demographic subgroup
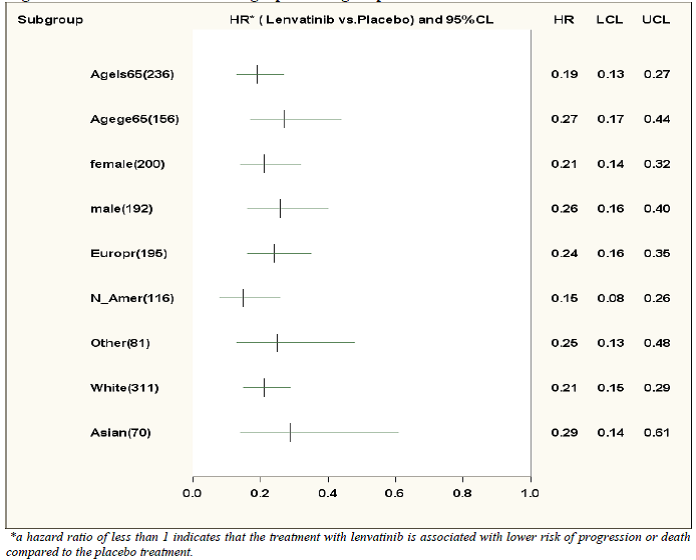
Extracted from Statistical Review, Figure 4.1
What are the possible side effects?
LENVIMA can cause serious side effects, including: high blood pressure (hypertension), heart problems, problems with blood clots in blood vessels (arteries), liver problems, increased protein in urine (proteinuria) and kidney failure, an opening in the wall of the stomach or intestines (perforation) or an abnormal connection between two parts of the gastrointestinal tract (fistula), changes in the electrical activity of the heart called QT prolongation that may lead to sudden death, low levels of blood calcium (hypocalcemia), low levels of calcium in the blood (hypocalcemia), the simultaneous occurrence of headache, confusion, seizures and visual changes (in a condition called Reversible Posterior Leukoencephalopathy Syndrome), serious bleeding (hemorrhage), risks to an unborn child if a patient becomes pregnant during treatment, and impairing suppression of the production of thyroid-stimulating hormone.
In the clinical trial, the most common side effects of LENVIMA were high blood pressure (hypertension), fatigue, diarrhea, joint and muscle pain (arthralgia/myalgia), decreased appetite, decreased weight, nausea, inflammation of the lining of the mouth (stomatitis), headache, vomiting, excess protein in the urine (proteinuria), swelling and pain in the palms, hands and/or the soles of the feet (called palmar-plantar erythrodysesthesia syndrome, or PPE), abdominal pain and changes in voice volume or quality (dysphonia).
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in at least 10% of the LENVIMA-treated group.
Table 4. Adverse Reactions Occurring in at Least 10% of LENVIMA-treated Patients
Adverse Reaction | LENVIMA 24 mg N=261 (%) | Placebo N=131 (%) |
|---|---|---|
| Hypertension | 73 | 16 |
| Diarrhea | 67 | 17 |
| Fatigue | 67 | 35 |
| Arthralgia/Myalgia | 62 | 28 |
| Decreased appetite | 54 | 18 |
| Weight decreased | 51 | 15 |
| Nausea | 47 | 25 |
| Stomatitis | 41 | 8 |
| Headache | 38 | 11 |
| Vomiting | 36 | 15 |
| Proteinuria | 34 | 3 |
| Palmar-plantar erythrodysesthesia | 32 | 1 |
| Abdominal pain | 31 | 11 |
| Dysphonia | 31 | 5 |
| Constipation | 29 | 15 |
| Oral pain | 25 | 2 |
| Cough | 24 | 18 |
| Edema peripheral | 21 | 8 |
| Rash | 21 | 3 |
| Dysgeusia | 18 | 3 |
| Dry mouth | 17 | 8 |
| Dizziness | 15 | 9 |
| Dyspepsia | 13 | 4 |
| Alopecia | 12 | 5 |
| Epistaxis | 12 | 1 |
| Insomnia | 12 | 3 |
| Urinary tract infection | 11 | 5 |
| Dental and oral infections | 10 | 1 |
Extracted from LENVIMA Package Insert, Table 2
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: Although the incidence of overall side effects was similar for men and women, certain side effects—called serious adverse events1—were observed more frequently in women compared to men. Other events (listed in MORE INFO) were observed more frequently in women compared to men.
- Race: Certain side effects (listed in MORE INFO) were seen more frequently in Whites compared with Asians, while others were seen less frequently. It is unclear if these differences are clinically meaningful. The number of non-White, non-Asians was limited and therefore differences could not be determined.
- Age: Subgroup analyses were conducted for ages below 65, ages 65 to 74, and 75 years and above. The incidence of overall side effects was similar across these age groups. Certain side effects—called serious adverse events1—were seen more frequently in patients 75 years and above.
1 Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect.
Table 5. Percentage of Subjects with AEs and SAEs by Sex
| LENVIMA N=261 % | Placebo N=131 % | |||
|---|---|---|---|---|
| Category | Male N=125 | Female N=136 | Male N=75 | Female N=56 |
| Subjects with an AE | 100 | 99 | 89 | 91 |
| Subjects with an SAE | 46 | 60 | 24 | 23 |
Extracted from Clinical Review, Table 64
Table 6. Adverse Events Observed More Frequently (at least 10% difference) in Females Compared to Males in LENVIMA-treated Patients
| Male N=200 | Female N=192 | |||
|---|---|---|---|---|
| LENVIMA N=125 n (%) | Placebo N=75 n (%) | LENVIMA N=136 n (%) | Placebo N=56 n (%) | |
| Hypertension | 79 (63.2) | 8 (10.7) | 102 (75) | 12 (21.4) |
| Headache | 40 (32) | 5 (6.7) | 60 (44.1) | 10 (17.9) |
| Nausea | 42 (33.6) | 18 (24) | 80 (58.8) | 15 (26.8) |
| Stomatitis | 38 (30.4) | 5 (6.7) | 58 (42.6) | 4 (7.1) |
| Fatigue | 46 (36.8) | 20 (26.7) | 65 (47.8) | 12 (21.4) |
| Proteinuria | 33 (26.4) | 3 (4.0) | 55 (40.4) | 1 (1.8) |
| Vomiting | 32 (25.6) | 12 (16) | 61 (44.9) | 7 (12.5) |
Extracted from Applicant’s Submission
Table 7. Adverse Events Observed More Frequently (at least 10% difference) in Asians Compared to Whites
| White | Asian | ||
|---|---|---|---|---|
LENVIMA | Placebo | LENVIMA | Placebo | |
Hypertension | 140 (67.3) | 12 (11.7) | 37 (80.4) | 4 (16.7) |
Peripheral edema | 37(17.8) | 7 (6.8) | 15 (32.6) | 2 (8.3) |
PPE | 51 (24.5) | 1 (1) | 28 (60.9) | 0 |
Thrombocytopenia | 9 (4.3) | 1 (1) | 14 (30.4) | 2 (8.3) |
PPE=Palmar-Plantar Erythrodysesthesia Syndrome
Extracted from Summary of Clinical Safety
Table 8. Adverse Events Observed More Frequently (at least 10% difference) in Whites Compared to Asians
| White | Asian | ||
|---|---|---|---|---|
LENVIMA | Placebo | LENVIMA | Placebo | |
Weight decreased | 112 (53.8) | 13 (12.6) | 16 (34.8) | 5 (20.8) |
Extracted from Summary of Clinical Safety
Table 9. Incidence of Adverse Events by Age Group
| LENVIMA | Placebo | ||||
|---|---|---|---|---|---|---|
Category | below 65 yrs | 65-75yrs> | ≥75yrs | 65yrs> | 65-75yrs> | ≥75yrs |
Subjects with an SAE | 75 (52) | 46 (52) | 18 (62) | 17 (22) | 13 (29) | 1 (11) |
Subjects with AE | 143 (100) | 89 (100) | 28 (97) | 67 (87) | 43 (96) | 8 (89) |
SAE=serious adverse event; AE=adverse event
Extracted from Clinical Review for LENVIMA, Table 63
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved LENVIMA based on evidence from one clinical trial of 392 patients with differentiated thyroid cancer (DTC) that was worsening and could no longer be treated with a treatment called radioactive iodine. The trial was conducted in North America, South America, Europe, Asia, Africa, and Australia.
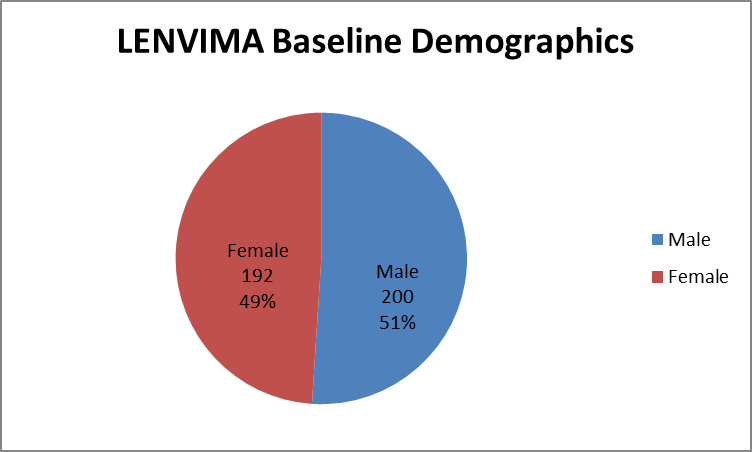
Figure 1 summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Extracted from Clinical review for LENVIMA, Table 12
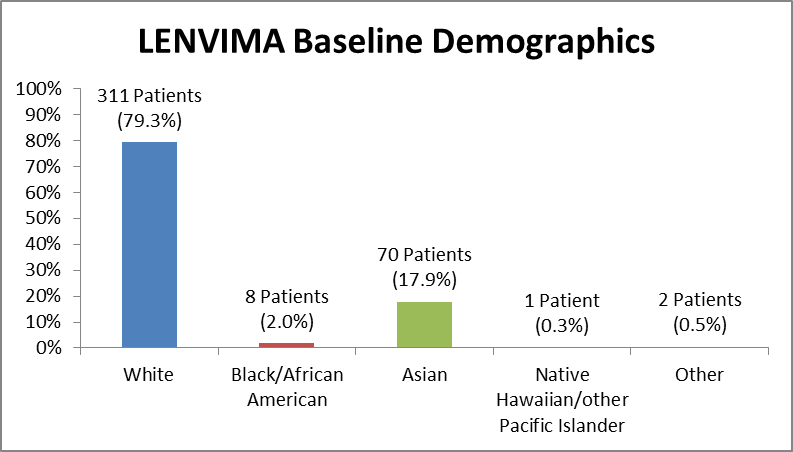
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Extracted from Clinical review for LENVIMA, Table 12
Table 1. Demographics of Trial by Race
Race | Number of Patients | Percentage |
|---|---|---|
White | 311 | 79.3% |
Black/African American | 8 | 2.0% |
Asian | 70 | 17.9% |
Native Hawaiian/other | 1 | 0.3% |
Other | 2 | 0.5% |
Extracted from Clinical review for LENVIMA, Table 12
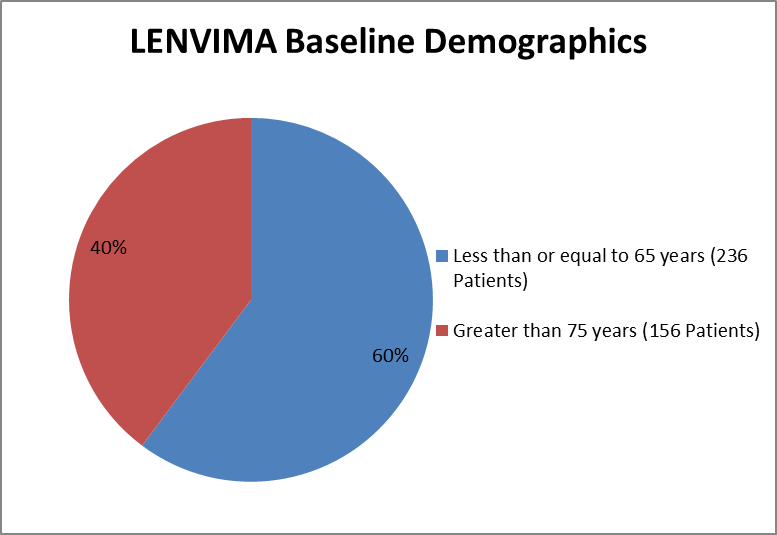
Figure 3 and Table 2 summarizes the percentage of patients enrolled in the clinical trial by age.
Figure 3. Baseline Demographics by Age
Extracted from Clinical review for LENVIMA, Table 12, page 56
Table 2. Baseline Demographics of Trial by Age
LENVIMA | Placebo | Total | |
|---|---|---|---|
Age (mean years) | 62.1 | 61.5 | 61.9 |
Age group n (%) | |||
Less than or equal to 65 years | 155 (59.4) | 81 (61.8) | 236 (60.2) |
Greater than 65 years | 106 (40.6) | 50 (38.2) | 156 (39.8) |
Extracted from Clinical review for LENVIMA, Table 12
Who participated in the trials?
LENVIMA was evaluated in one clinical trial in 392 patients with locally recurrent or metastatic radioactive iodine-refractory differentiated thyroid cancer and evidence of disease progression within 12 months prior to randomization on scans, confirmed by independent review by a radiology physician. The trial was conducted in North America, South America, Europe, Asia, Africa, and Australia.
Table 10. Demographic and Baseline Characteristics (Intent to Treat Population)
Variable | LENVIMA | Placebo | Total |
|---|---|---|---|
Age (year) | |||
Mean | 62.1 | 61.5 | 61.9 |
Median | 64 | 61 | 63 |
Age group n (%) |
|
|
|
≤ 65yrs | 155 (59.4) | 81 (61.8) | 236 (60.2) |
> 65yrs | 106 (40.6) | 50 (38.2) | 156 (39.8) |
Sex n (%) | |||
Male | 125 (47.9) | 75 (57.3) | 200 (51) |
Female | 136 (52.1) | 56 (42.7) | 192 (49) |
Region n (%) | |||
Europe | 131 (50.2) | 64 (48.9) | 195 (49.7) |
North America | 77 (29.5) | 39 (29.8) | 116 (29.6) |
Other | 53 (20.3) | 28 (21.4) | 81 (20.7) |
Race n (%) |
|
|
|
White | 208 (79.7) | 103 (78.6) | 311 (79.3) |
Black/African American | 4 (1.5) | 4 (3.1) | 8 (2.0) |
Asian | 46 (17.6) | 24 (18.3) | 70 (17.9) |
Japanese | 30 (11.5) | 11 (8.4) | 41 (10.5) |
Other Asian | 16 (6.1) | 13 (9.9) | 29 (7.4) |
Native Hawaiian/other | 1 (0.4) | 0 | 1 (0.3) |
Other | 2 (0.8) | 0 | 2 (0.5) |
Source: Clinical review for LENVIMA, Table 12, page 56
How were the trials designed?
In the trial, two-thirds of the patients were chosen at random to receive LENVIMA and one-third were given a placebo. Neither the patients nor the health care professionals administering the drug knew which patients were taking LENVIMA and which ones were taking a placebo. They remained in these groups until their disease began to get worse. If the disease progressed, those taking placebo were allowed to receive LENVIMA.
How were the trials designed?
Efficacy of LENVIMA was evaluated in a multicenter, randomized (2:1), double-blind, placebo-controlled trial in 392 patients with locally recurrent or metastatic radioactive iodine-refractory differentiated thyroid cancer and radiographic evidence of disease progression within 12 months prior to randomization, confirmed by independent radiologic review.
Patients were randomized to receive LENVIMA 24 mg once daily or placebo until disease progression. Randomization was stratified by geographic region, prior VEGF/VEGFR-targeted therapy, and age.
The major efficacy outcome measure was progression-free survival as determined by blinded independent radiologic review using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PACKAGE INSERT
MEDICAL REVIEW