Drug Trials Snapshot: TUKYSA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TUKYSA Package Insert for complete information.
TUKYSA (tucatinib)
(too kye' sah)
Seattle Genetics
Approval date: April 17, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TUKYSA is a drug for treatment of adults with human epidermal growth factor receptor (HER)2-positive breast cancer that has spread to other parts of the body including the brain (metastatic) or cannot not be removed by surgery.
It should be used in patients who have been previously treated for their metastatic disease with at least one anti-HER2 regimen and in combination with two other medications for the treatment of metastatic breast cancer (trastuzumab and capecitabine).
How is this drug used?
TUKYSA is a tablet taken by mouth two times a day in combination with trastuzumab and capecitabine.
What are the benefits of this drug?
The trial measured the length of time tumors did not grow after treatment (progression-free survival or PFS). The progression-free survival for patients taking TUKYSA together with trastuzumab and capecitabine was about 8 months compared to 6 months for patients taking a placebo with trastuzumab and capecitabine.
What are the benefits of this drug?
Table 1 shows efficacy results based on the progression-free survival in the first 480 randomized patients assessed by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Additional efficacy outcome measures were evaluated in all randomized patients and included overall survival, PFS among patients with a history or presence of brain metastases and confirmed objective response rate.
Table 1. Efficacy Results
| PFS1 | TUKYSA + Trastuzumab + Capecitabine | Placebo + Trastuzumab + Capecitabine |
|---|---|---|
| N=320 | N=160 | |
| Number of events (%) | 178 (56) | 97 (61) |
| Median (months) (95% CI) |
7.8 (7.5, 9.6) |
5.6 (4.2, 7.1) |
| Hazard ratio (95% CI)2 |
0.54 (0.42, 0.71) |
|
| P-value3 | <0.00001 | |
BICR=blinded independent central review; CI=confidence interval; PFS=progression-free survival.
- Primary PFS analysis conducted in first 480 randomized patients.
- Hazard ratio and 95% confidence intervals are based on stratified Cox proportional hazards regression model controlling for stratification factors (presence or history of brain metastases, ECOG status, and region of world)
- Two-sided p-value based on re-randomization procedure (Rosenberger and Lachin 2002) controlling for stratification factors, compared with the allocated alpha of 0.05
Adapted from TUKYSA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Almost all included patients were women, therefore sex differences could not be determined.
- Race: The majority of patients were White, therefore race differences could not be determined.
- Age: TUKYSA worked similarly in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
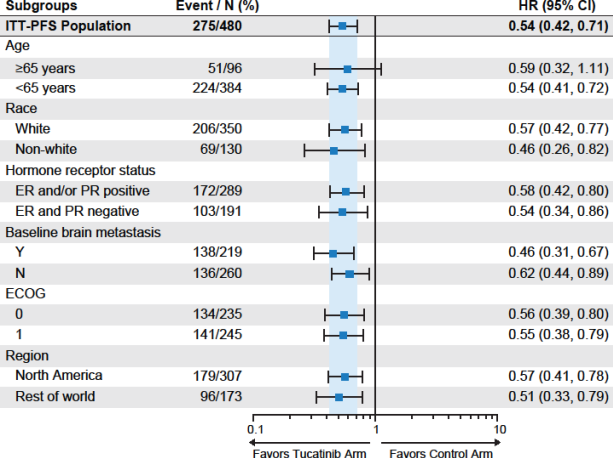
Figure 5 shows the efficacy results by relevant demographic subgroups.
Figure 5. PFS per BIRC by Subgroups (ITT-PFS population)
Hazard ratio was calculated from cox regression model considering stratified factors from randomization. ITT=intent-to treat-population
'Race Non-White' included subjects with race other than white.
‘Hormone receptor status: ER and PR negative’ included subjects without positive estrogen or positive progesterone.
‘Baseline brain metastasis: Y’ included subjects with a history of brain metastases or presence of brain metastases or brain lesions of equivocal significance on screening MRI per EDC data.
FDA Review
What are the possible side effects?
TUKYSA may cause serious side effects including severe diarrhea, liver damage, and harm to an unborn baby.
The most common side effects of TUKYSA are diarrhea, palmar-plantar erythrodysesthesia (rash, redness, pain, swelling or blisters on the palms of the hands or soles of the feet) nausea, tiredness, liver toxicity, vomiting and mouth sores.
What are the possible side effects?
Table 3 below summarizes common adverse reactions.
Table 3. Adverse Reactions (≥10%) in Patients Who Received TUKYSA and with a Difference Between Arms of ≥ 5% Compared to Placebo (All Grades)
| Adverse Reaction | TUKYSA + Trastuzumab +Capecitabine N = 404 |
Placebo + Trastuzumab + Capecitabine N = 197 |
||||
|---|---|---|---|---|---|---|
| All Grades % |
Grade 3 % |
Grade 4 % |
All Grades % |
Grade 3 % |
Grade 4 % |
|
| Gastrointestinal disorders | ||||||
| Diarrhea | 81 | 12 | 0.5 | 53 | 9 | 0 |
| Nausea | 58 | 3.7 | 0 | 44 | 3 | 0 |
| Vomiting | 36 | 3 | 0 | 25 | 3.6 | 0 |
| Stomatitis1 | 32 | 2.5 | 0 | 21 | 0.5 | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Palmar-plantar erythrodysesthesia syndrome | 63 | 13 | 0 | 53 | 9 | 0 |
| Rash2 | 20 | 0.7 | 0 | 15 | 0.5 | 0 |
| Hepatobiliary disorders | ||||||
| Hepatotoxicity3 | 42 | 9 | 0.2 | 24 | 3.6 | 0 |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 25 | 0.5 | 0 | 20 | 0 | 0 |
| Blood and lymphatic system disorders | ||||||
| Anemia4 | 21 | 3.7 | 0 | 13 | 2.5 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||
| Arthralgia | 15 | 0.5 | 0 | 4.6 | 0.5 | 0 |
| Investigations | ||||||
| Creatinine increased5 | 14 | 0 | 0 | 1.5 | 0 | 0 |
| Weight decreased | 13 | 1 | 0 | 6 | 0.5 | 0 |
| Nervous System Disorders | ||||||
| Peripheral neuropathy6 | 13 | 0.5 | 0 | 7 | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Epistaxis | 12 | 0 | 0 | 5 | 0 | 0 |
- Stomatitis includes stomatitis, oropharyngeal pain, oropharyngeal discomfort, mouth ulceration, oral pain, lip ulceration, glossodynia, tongue blistering, lip blister, oral dysesthesia, tongue ulceration, and aphthous ulcer
- Rash includes rash maculo-papular, rash, dermatitis acneiform, erythema, rash macular, rash papular, rash pustular, rash pruritic, rash erythematous, skin exfoliation, urticaria, dermatitis allergic, palmar erythema, plantar erythema, skin toxicity, and dermatitis
- Hepatotoxicity includes hyperbilirubinemia, blood bilirubin increased, bilirubin conjugated increased, alanine aminotransferase increased, transaminases increased, hepatotoxicity, aspartate aminotransferase increased, liver function test increased, liver injury, and hepatocellular injury
- Anemia includes anemia, hemoglobin decreased, and normocytic anemia
- Due to inhibition of renal tubular transport of creatinine without affecting glomerular function
- Peripheral neuropathy includes peripheral sensory neuropathy, neuropathy peripheral, peripheral motor neuropathy, and peripheral sensorimotor neuropathy
TUKYSA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Almost all included patients were women therefore sex differences could not be determined.
- Race: The majority of patients were White, therefore race differences in side effects could not be determined.
- Age: The occurrence of overall side effects was similar in patients above and below 65 years of age. The occurrence of serious side effects1 was higher in patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analysis of side effects by age subgroups is presented below. Race and sex analyses were not conducted because of predominance of women and Whites.
Table 5. Summary of Treatment-Emergent Adverse Events (TEAEs) by Age
| Adverse Event | TUKYSA+ Trastuzumab + Capecitabine N=404 |
Placebo + Trastuzumab + Capecitabine N = 197 |
||
|---|---|---|---|---|
| <65y N=322 |
≥65y N=82 |
<65y N=164 |
≥65y N=33 |
|
| Any TEAEs, n (%) | 320 (99) | 81 (99) | 150 (97) | 32 (97) |
| Grade 3-4, n (%) | 172 (53) | 43 (53) | 70 (43) | 20 (61) |
| SAEs, n (%) | 76 (24) | 28 (34) | 41 (25) | 12 (36) |
SAEs=serious adverse events
FDA Review and Clinical Trial Report
1Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved TUKYSA based on evidence from one clinical trial (NCT02614794) of patients with HER2-positive metastatic breast cancer. The trial was conducted at 157 sites in the United States, Canada, Europe, Israel and Australia.
Presented below is safety population (601 patients) which includes all patients from the trail who received at least one dose of TUKYSA and provided data for assessment of side effects.
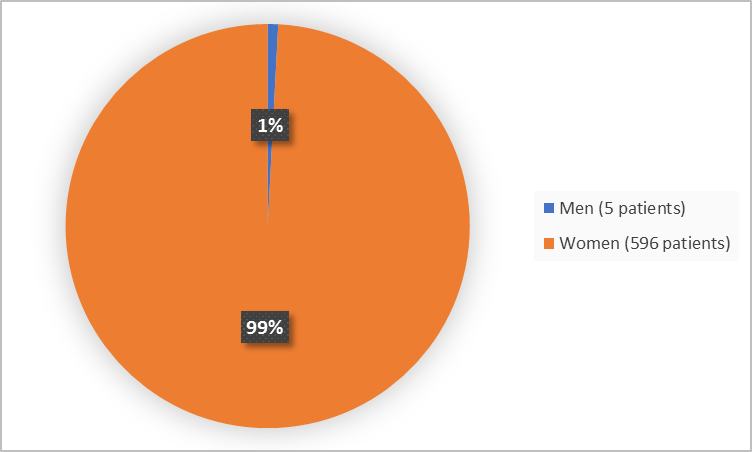
Figure 1 summarizes by sex how many patients were in the clinical trial.
Figure 1. Baseline Demographics by Sex (safety population)
Adapted from FDA Review
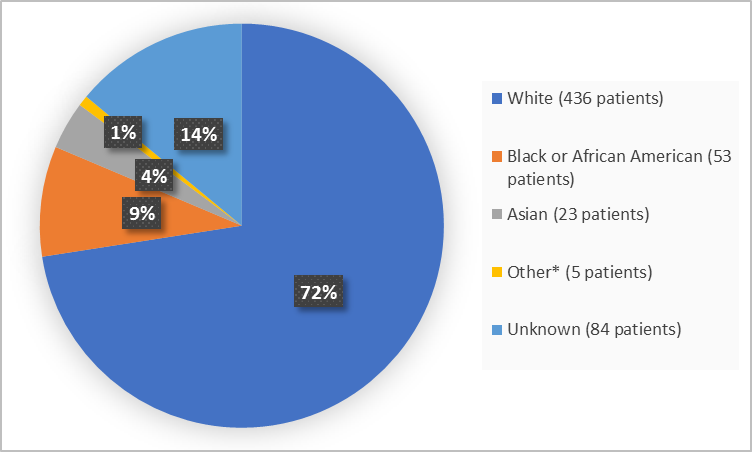
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (safety population)
*includes Native Hawaiian or Other Pacific Islander and Multiple
Adapted from FDA Review
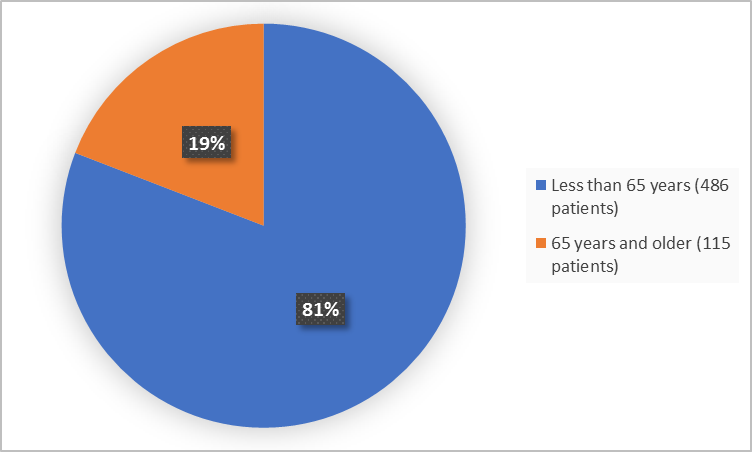
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age (safety population)
Adapted from FDA Review
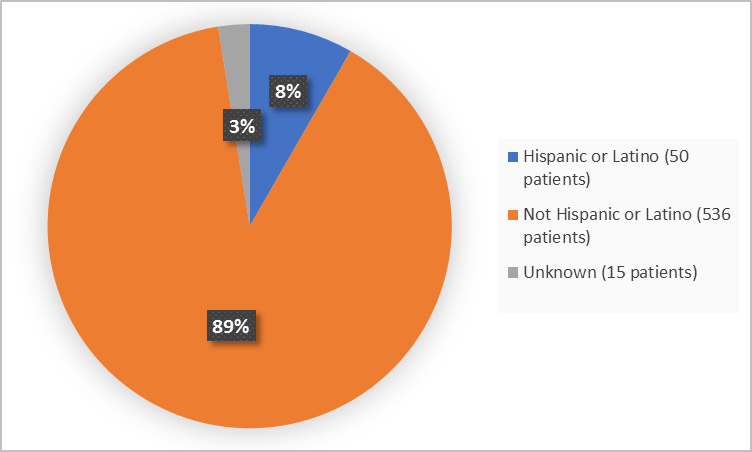
Figure 4 summarizes patients by ethnicity in the clinical trial.
Figure 4. Baseline Demographics by Ethnicity (safety population)
Adapted from FDA Review
Who participated in the trials?
The demographic characteristics of the safety population are summarized in Table 6.
Table 6. Demographic Characteristics (safety population)
| TUKYSA + Trastuzumab + Capecitabine N=404 |
Placebo + Trastuzumab + Capecitabine N=197 |
TOTAL N=601 | |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 3 (0.7) | 2 (1.0) | 5 (0.8) |
| Women | 401 (99.3) | 195 (99.0) | 596 (99.2) |
| Race, n (%) | |||
| White | 283 (70.0) | 153 (77.7) | 436 (72.5) |
| Black or African American | 39 (9.7) | 14 (7.1) | 53 (8.8) |
| Asian | 18 (4.5) | 5 (2.5) | 23 (3.8) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2) | 0 | 1 (0.2) |
| Multiple | 2 (0.5) | 2 (1.0) | 4 (0.7) |
| Unknown | 61 (15.1) | 23 (11.7) | 84 (14.0) |
| Age, years | |||
| Median | 55.0 | 54.0 | 54 |
| Min, Max | 22, 80 | 25, 78 | 22, 80 |
| Age Category, n (%) | |||
| <65 years | 322 (79.7) | 164 (83.2) | 486 (80.9) |
| ≥65 years | 82 (20.3) | 33 (16.8) | 115 (19.1) |
| ≥75 years | 8 (2.0) | 4 (2.0) | 12 (2.0) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 36 (8.9) | 14 (7.1) | 50 (8.3) |
| Not Hispanic or Latino | 357 (88.4) | 179 (90.9) | 536 (89.2) |
| Unknown | 11 (2.7) | 4 (2) | 15 (2.5) |
| Geographic Region, n (%) | |||
| USA | 215 (53.2) | 107 (54.3) | 322 (53.6) |
| Rest of World | 189 (46.8) | 90 (45.7) | 279 (46.4) |
Adapted from FDA Review
How were the trials designed?
There was one trial that provided data for TUKYSA approval. The trial enrolled adult patients with HER2-positive metastatic breast cancer who have been treated presviously for their metastatic disease.
Patients received TUKYSA or placebo twice daily, in combination with trastuzumab and capecitabine. The treatment continued until the disease progressed or the side effects became too toxic.
The trial measured the length of time tumors did not grow (progression-free survival).
How were the trials designed?
There was one trial that provided data for approval of TUKYSA.
Enrolled patients were required to have HER2-positive, unresectable locally advanced or metastatic breast cancer, with or without brain metastases, and had prior treatment with trastuzumab, pertuzumab, and ado-trastuzumab emtansine (T-DM1) separately or in combination, in the neoadjuvant, adjuvant or metastatic setting.
Patients received TUKYSA 300 mg or placebo, orally twice daily, in combination with a trastuzumab loading dose of 8 mg/kg on Day 1 of Cycle 1 if needed and then a maintenance dose of 6 mg/kg on Day 1 of every 21-day cycle, and capecitabine 1000 mg/m2 orally twice daily on Days 1 through 14 of every 21-day cycle. An alternate trastuzumab dosing regimen was 600 mg administered subcutaneously on Day 1 of every 21-day cycle. Patients were treated until disease progression or unacceptable toxicity. Tumor assessments, including brain-MRI in patients with presence or history of brain metastases at baseline, occurred every 6 weeks for the first 24 weeks and every 9 weeks thereafter. The major efficacy outcome measure was progression-free survival (PFS) in the first 480 randomized patients assessed by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Additional efficacy outcome measures were evaluated in all randomized patients and included overall survival, PFS among patients with a history or presence of brain metastases, and confirmed objective response rate (ORR).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION