Drug Trials Snapshot: Venclexta
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to VENCLEXTA Prescribing Information for complete information.
VENCLEXTA (venetoclax)
(ven-KLEKS-tuh)
AbbVie Inc. & Genentech USA, Inc.
Approval date: April 11, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VENCLEXTA is a drug for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a chromosomal abnormality called 17p deletion and who have been treated with at least one prior therapy.
How is this drug used?
VENCLEXTA is a tablet taken by mouth once a day. The starting dose is slowly increased over 5 weeks up to the full dose.
What are the benefits of this drug?
Results showed that 80 percent of patients achieved complete or partial remission of their cancer.
What are the benefits of this drug (results of trial used to assess efficacy)?
The table below summarizes efficacy results for the Trial 1.
Table 2. Efficacy Results for Patients with Previously Treated CLL with 17p Deletion by IRC
| VENCLEXTA N=106 |
|
|---|---|
| ORR, n (%) (95% CI) |
85 (80.2) (71.3, 87.3) |
| CR + CRi, n (%) CR, n (%) CRi, n (%) |
8 (7.5) 6 (5.7) 2 (1.9) |
| nPR, n (%) | 3 (2.8) |
| PR, n (%) | 74 (69.8) |
| CI = confidence interval; CR = complete remission; CRi = complete remission with incomplete marrow recovery; IRC = independent review committee; nPR = nodular partial remission; ORR = overall response rate (CR + CRi + nPR + PR); PR = partial remission. | |
Demographic data for efficacy population for Trial 1 is presented below:
Table 3. Baseline Demographics of Patients in the Trial 1 (Efficacy Population)
| Demographic Parameters | VENCLEXTA N=106 n (%) |
|---|---|
| Sex | |
| Men | 69 (65) |
| Women | 37 (35) |
| Age Group | |
| < 65=""> | 46 (43) |
| ≥ 65 years | 60 (57) |
| Race | |
| White | 102 (97) |
| Black or African American | 3 (3) |
| Missing | 1 (1) |
| Region | |
| United States | 17 (16) |
| Europe | 79 (75) |
| Rest of the world | 10 (9) |
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: VENCLEXTA worked similarly in men and women.
- Race: The majority of participants in the clinical trials were white. Differences among races could not be determined due to small number of participants in other races.
- Age: VENCLEXTA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figure below summarizes efficacy results by subgroup for the Trial 1.
Table 4 . Subgroup Analysis of IRC Assessed ORR by Demographics
| Subgroup | Number of patients |
IRC assessment n (%) (95% CI)a |
|---|---|---|
| Sex | ||
| Male | 69 | 56 (81) (69.9,89.6) |
| Female | 37 | 29 (78.4) (61.8, 90.2) |
| Age group | ||
| Age <65> | 46 | 40 (87) (73.7, 95.1) |
| Age ≥65 years | 60 | 45 (75) (62.1,85.3) |
| Race | ||
| White | 102 | 81 (79.4) (70.3,86.8) |
| Black | 3 | 3 (100) |
| Other | 1 | 1 (100) |
IRC = independent review committee
ORR = overall response rate
CI=confidence interval
a 95% CI was based on Clopper-Pearson exact method
Clinical trial data
What are the possible side effects?
The most common side effects are low white blood cell count (neutropenia), diarrhea, nausea, low red blood cell count (anemia), upper respiratory tract infection, low platelet count (thrombocytopenia), and fatigue.
VENCLEXTA may cause serious side effects including neutropenia with fever and metabolic abnormalities known as tumor lysis syndrome.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with VENCLEXTA (pooled Trials 1, 2 and 3).
Table 5. Adverse Reactions Reported in ≥10% (Any Grade) or ≥5% (Grade 3 or 4) of CLL Patients
| Body System | Adverse Reaction | Any Grade (%) N=240 |
Grade 3 or 4 (%) N=240 |
|---|---|---|---|
| Blood and lymphatic system disorders | Neutropeniaa | 45 | 41 |
| Anemiab | 29 | 18 | |
| Thrombocytopeniac | 22 | 15 | |
| Febrile neutropenia | 5 | 5 | |
| Gastrointestinal disorders | Diarrhea | 35 | <> |
| Nausea | 33 | <> | |
| Vomiting | 15 | <> | |
| Constipation | 14 | 0 | |
| General disorders and administration site conditions | Fatigue | 21 | 2 |
| Pyrexia | 16 | <> | |
| Peripheral edema | 11 | <> | |
| Infections and infestations | Upper respiratory tract infection | 22 | 1 |
| Pneumonia | 8 | 5 | |
| Metabolic and nutrition disorders | Hypokalemia | 12 | 4 |
| Musculoskeletal and connective tissue disorders | Back pain | 10 | <> |
| Nervous system disorders | Headache | 15 | <> |
| Respiratory, thoracic, and mediastinal disorders | Cough | 13 | 0 |
| Adverse Reactions graded using NCI Common Terminology Criteria for Adverse Events version 4.0. aNeutropenia/neutrophil count decreased. bAnemia/hemoglobin decreased. cThrombocytopenia/platelet count decreased. |
|||
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of participants in the clinical trials were white. Differences in side effects among races could not be determined due to small number of participants in other races.
- Age: The occurrence of side effect was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes TEAEs (Treatment-Emergent Adverse Events) in pooled Trials 1, 2 and 3 by subgroup.
Table 6. TEAEs by Subgroup (Safety Population)
| Any TEAE | VENCLEXTA (N=240) n/N (%) |
|---|---|
| Sex | |
| Men | 162/166 (98) |
| Women | 74/74 (100) |
| Age Group | |
| ≤65 years | 100/102 (98) |
| >65 years | 136/138(97) |
| Race | |
| White | 221/225 (98) |
| Non-White | 15/15 (100) |
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved VENCLEXTA based on evidence from clinical trials that evaluated the benefits and the side effects of VENCLEXTA.
The FDA evaluated benefits of VENCLEXTA based on evidence from one clinical trial (Trial 1) of 106 patients with CLL who had a 17p deletion and who had received at least one prior therapy. The trial was conducted in the USA, Canada, France, Germany, Poland, United Kingdom and Australia. Patients who participated in this trial are described in Table 3 in the section MORE INFO.
The FDA evaluated side effects of VENCLEXTA based on evidence from three clinical trials (Trials 1, 2 and 3) of 240 patients who received VENCLEXTA at the recommended dose. Trials were conducted in the USA, Canada, France, Germany, Poland, United Kingdom and Australia.
The figure below summarizes how many men and women were in the clinical trials used to evaluate the side effects of VENCLEXTA (also called the Safety Population).
Figure 1. Baseline Demographics by Sex (Safety Population)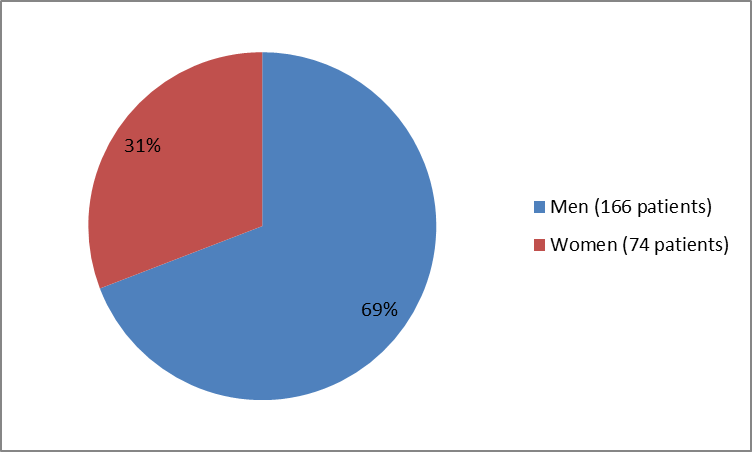
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trials used to evaluate the side effects of VENCLEXTA.
Figure 2. Baseline Demographics by Race (Safety Population)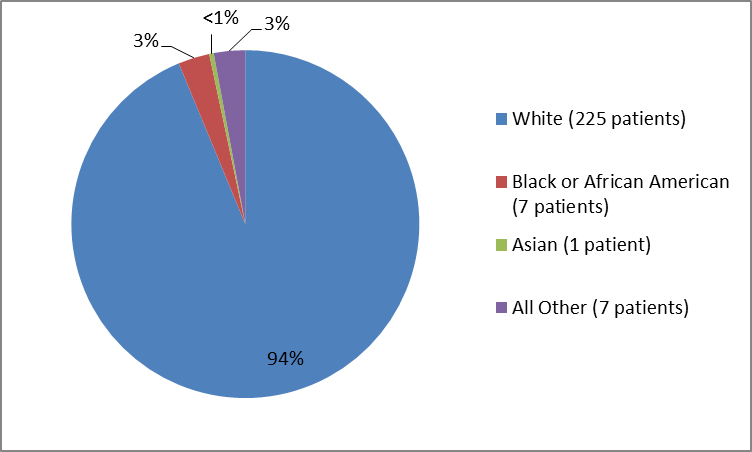
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
| White | 225 | 94% |
| Black or African American | 7 | 3% |
| Asian | 1 | less than 1% |
| Multi-racial | 1 | less than 1% |
| Other | 4 | 2% |
| Missing | 2 | 1% |
Figure 3 summarizes the percentage of patients by age group in the clinical trials used to evaluate the side effects of VENCLEXTA.
Figure 3. Baseline Demographics by Age (Safety Population)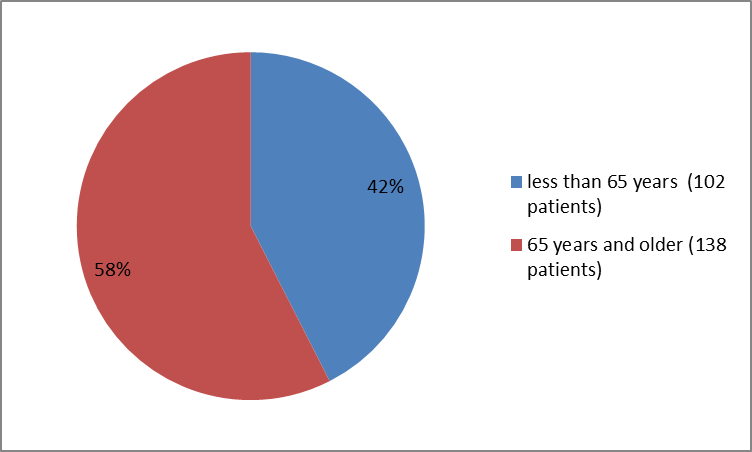
Who participated in the trials?
The table below summarizes demographics of patients in pooled Trials 1, 2 and 3.
Table 7. Baseline Demographics of Patients in the Clinical Trials (Safety Population)
| Demographic Parameters | VENCLEXTA (N=240) n (%) |
|---|---|
| Sex | |
| Men | 166 (69) |
| Women | 74 (31) |
| Age | |
| Mean years (SD) | 65.3 (9.37) |
| Median (years) | 66 |
| Min, max (years) | 29, 85 |
| Age Group | |
| < 65 years | 102 (43) |
| ≥ 65 years | 138 (57) |
| Race | |
| White | 225 (95) |
| Black or African American | 7(3) |
| Asian | 1 (<> |
| Multi-racial | 1 (<> |
| Other | 4 (2) |
| Missing | 2 (1) |
| Region | |
| United States | 106 (44) |
| Europe | 94 (39) |
| Rest of the world | 40 (17) |
How were the trials designed?
The benefits of VENCLEXTA were evaluated in one clinical trial of 106 patients who had 17p deletion and who had received at least one prior therapy. All patients received treatment with VENCLEXTA until disease progression or unacceptable side effect.
The benefit of VENCLEXTA was evaluated by measuring the proportion of patients who achieved either complete or partial remission of the disease.
The safety of VENCLEXTA was evaluated in three clinical trials of 240 patients who received VENCLEXTA at recommended dose.
How were the trials designed?
The efficacy of VENCLEXTA was established in an open-label, single-arm, multicenter clinical trial of 106 patients with CLL with 17p deletion who had received at least one prior therapy. Patients received VENCLEXTA orally once daily until disease progression or unacceptable toxicity.
The efficacy of VENCLEXTA was evaluated by overall response rate (ORR) as assessed by an Independent Review Committee (IRC) using the International Workshop for Chronic Lymphocytic Leukemia (IWCLL) updated National Cancer Institute-sponsored Working Group (NCI-WG) guidelines.
The safety of VENCLEXTA at the recommended daily dose was evaluated on pooled data of 240 patients with CLL treated with VENCLEXTA in two phase 2 trials and one phase 1 trial.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PACKAGE INSERT
