Drug Trials Snapshots: ASPARLAS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ASPARLAS Package Insert for complete information.
ASPARLAS (calaspargase pegol-mknl)
a-spar-las
Baxalta US, Inc
Approval date: December 20, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ASPARLAS is used in combination with other drugs for the treatment of acute lymphoblastic leukemia (ALL) in patients 1 month to 21 years old.
ALL is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of lymphocytes (a type of white blood cells) in the bloodstream.
How is this drug used?
ASPARLAS is given by a healthcare provider directly into the bloodstream through a needle in the vein (known as an intravenous or IV infusion) no more frequently than every 21 days. It takes about one hour to receive an ASPARLAS infusion.
What are the benefits of this drug?
Leukemia cells depend on asparagine in the blood for survival. Without adequate blood levels of asparagine, leukemia cells die.
Ninety-nine percent of the 123 patients who received ASPARLAS demonstrated removal of asparagine from the blood at 6, 12, 18, 24, and 30 weeks after treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
The figure below summarizes efficacy results for the evaluated patients in the trial. The primary outcome was achievement and maintenance of nadir serum asparaginase activity (NSAA) above the level of 0.1 U/mL.
Figure 4. NSAA in Patients with B Cell Lineage ALL
ASPARLAS Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ASPARLAS worked similarly between males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how well the drug worked among races could not be determined.
- Age: The majority of patents were children. ASPARLAS worked similarly between tested groups. Differences in how well the drug worked between children and adults could not be determined because of the limited number of adults.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The table below summarizes efficacy results by sex, and age based on NSAA > 0.1 U/mL at weeks 6, 12, 18, 24 and 30. The majority of patients were White; therefore, subgroup analyses for race were not performed.
Table 2. Subgroup Analysis of NSAA >0.1 U/mL (efficacy population)
|
Demographic Characteristic |
ASPARLAS |
|---|---|
|
Sex |
|
|
Men |
61/123 (49.6%) |
|
Women |
62/123 (50.4%) |
|
Age Group |
|
|
≤ 10 years |
55/123 (44.7%) |
|
> 10 years |
68/123 (55.3%) |
FDA Review
What are the possible side effects?
ASPARLAS may cause serious and life threating allergic reactions and blood clots. Other serious side effects include inflammation in the pancreas and liver damage.
The most common side effects of ASPARLAS are increase in liver tests, inflammation of the pancreas, and abnormal blood clotting tests.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with ALL or lymphoblastic lymphoma in the clinical trial (safety population).
Table 3. Selected Grades ≥3 Adverse Reactions in Patients Receiving ASPARLAS with Multi-Agent Chemotherapy (Trial 1)*,†
|
Adverse Reaction‡
|
ASPARLAS |
Pegaspargase |
|---|---|---|
|
Grades ≥3 |
Grades ≥3 |
|
|
Elevated transaminase |
61 (52) |
79 (66) |
|
Bilirubin increased |
24 (20) |
30 (25) |
|
Pancreatitis |
21 (18) |
29 (24) |
|
Abnormal clotting studies |
17 (14) |
25 (21) |
|
Diarrhea |
10 (9) |
6 (5) |
|
Hypersensitivity |
9 (8) |
8 (7) |
|
Embolic and thrombotic events |
9 (8) |
10 (8) |
|
Sepsis |
6 (5) |
7 (6) |
|
Dyspnea |
5 (4) |
1 (1) |
|
Hemorrhages |
5 (4) |
5 (4) |
|
Fungal infection |
4 (3) |
3 (3) |
|
Pneumonia |
4 (3) |
8 (7) |
|
Arrhythmia |
2 (2) |
1 (1) |
|
Cardiac failure |
2 (2) |
1 (1) |
* ASPARLAS or pegaspargase were administered as a component of multi agent chemotherapy regimens
‡ Grouped terms: Elevated transaminase: Alanine aminotransferase increased, Aspartate aminotransferase increased, Transaminases increased; Bilirubin increased: Bilirubin conjugated increased, Blood bilirubin increased; Pancreatitis: Amylase increased, Lipase increased, Pancreatic necrosis, Pancreatitis, Pancreatitis relapsing; Abnormal clotting studies: Activated partial thromboplastin time prolonged, Blood fibrinogen decreased; Diarrhea: Colitis, Diarrhea, Enterocolitis, Neutropenic colitis; Hypersensitivity: Anaphylactic reaction, Drug hypersensitivity, Hypersensitivity; Embolic and thrombotic events SMQ: Device related thrombosis, Disseminated intravascular coagulation, Embolism, Intracardiac thrombus, Intracranial venous sinus thrombosis, Pulmonary embolism, Superior sagittal sinus thrombosis, Thrombosis in device, Venous thrombosis, Venous thrombosis limb; Sepsis: Bacterial sepsis, Sepsis; Dyspnea: Hypoxia, Respiratory failure; Hemorrhages SMQ (excludes laboratory terms): Disseminated intravascular coagulation, Epistaxis, Hematoma, Hemorrhage intracranial, Melena, Esophageal ulcer hemorrhage, Small intestinal hemorrhage, Upper gastrointestinal hemorrhage; Fungal infection: Fungal infection, Hepatic infection fungal, Respiratory tract infection fungal, Splenic infection fungal, Systemic candida; Pneumonia: Lung infection, Pneumonia, Pneumonitis; Arrhythmia: Atrioventricular block complete, Sinus tachycardia, Ventricular arrhythmia; Cardiac failure: Ejection fraction decreased, Left ventricular dysfunction.
§ Grading is based on the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
ASPARLAS Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among other races could not be determined.
- Age: The majority of patients were children. The occurrence of side effects was similar among tested groups. However, the number of adult patents was limited; therefore, differences in the occurrence of side effects between children and adult patients could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes the occurrence of the most common adverse reaction, elevated transaminase, by subgroup.
Table 4. Subgroup Analysis of Elevated Transaminasea (safety population)
|
Demographic Characteristic |
ASPARLAS |
pepaspargase |
|---|---|---|
|
Sex |
||
|
Male |
40/75 (53) |
51/71 (72) |
|
Female |
21/43 (49) |
28/48 (58) |
|
Race |
||
|
White |
43/78 (55) |
61/89 (69) |
|
Black or African American |
4/9(44) |
1/2 (50) |
|
Asian |
3/6 (50) |
3/4 (75) |
|
Other |
11/23 (48) |
14/24 (58) |
|
Age Group |
||
|
1 to 10 years |
46/90 (51) |
58/92 (63) |
|
11 to 15 years |
12/22 (55) |
16/20 (80) |
|
16 to 20 |
3/6 (50) |
5/7 (71) |
aElevated Transaminase includes alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased.
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ASPARLAS based on evidence primarily from two clinical trials (Trial 1/NCT01574274 and Trial 2/AALL07P4) of 237 patients with acute lymphoblastic leukemia (ALL). The trials were conducted in the United States and Canada.
Demographics of the patients who provided data for evaluation of benefits (efficacy population) are presented in Table 6, under the MORE INFO section.
The population that provided data for side effects of ASPARLAS (safety population) is presented below.
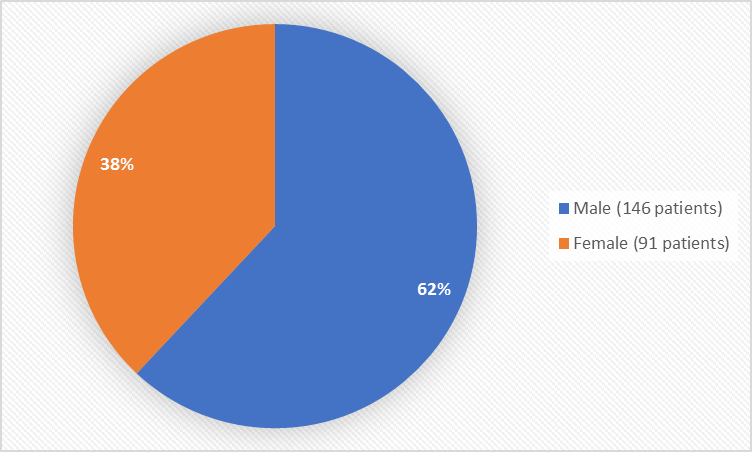
Figure 1 summarizes how many males and females were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
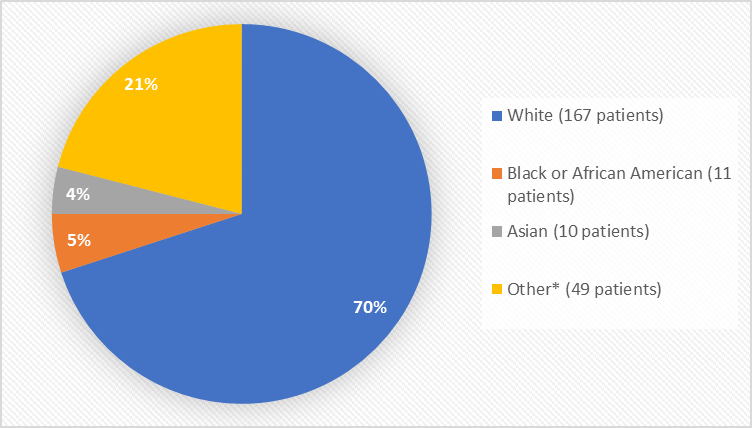
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
*Other includes Native Hawaiian or Other Pacific Islander
Table 1. Demographics of Efficacy Trials by Race
|
Race |
Number of Patients |
Percentage of Patients |
|---|---|---|
|
White |
167 |
70% |
|
Black or African American |
11 |
5% |
|
Asian |
10 |
4% |
|
Native Hawaiian or Other Pacific Islander |
2 |
1% |
|
Other |
47 |
20% |
Clinical Trial Data
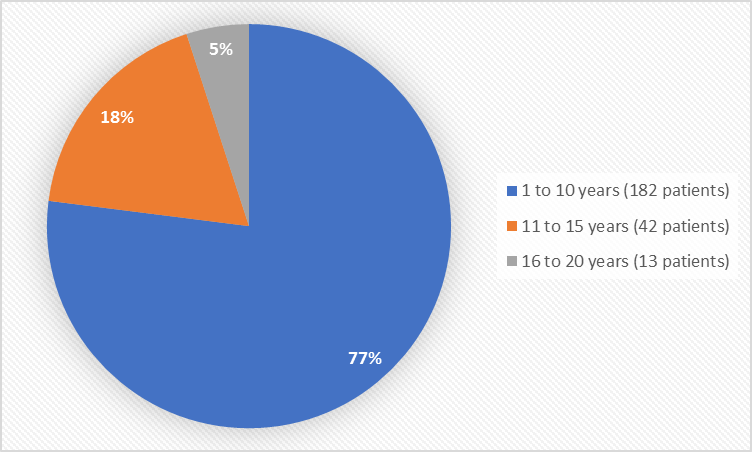
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The tables below summarize demographics of the safety and efficacy populations.
Table 5. Demographics of the Safety Population
|
Demographic Characteristics |
ASPARLAS |
pepaspargase |
Total |
|---|---|---|---|
|
Sex n (%) |
|||
|
Male |
75 (64) |
71 (60) |
146 (62) |
|
Female |
43 (36) |
48 (40) |
91 (38) |
|
Race n (%) |
|||
|
White |
78 (66) |
89 (75) |
167 (70) |
|
Black or African American |
9 (8) |
2 (2) |
11 (5) |
|
Asian |
6 (5) |
4 (3) |
10 (4) |
|
Native Hawaiian or Other Pacific Islander |
2 (2) |
0 (0) |
2 (1) |
|
Other |
23 (19) |
24 (20) |
47 (20) |
|
Age Group n (%) |
|||
|
1 – 10 years |
90 (76) |
92 (78) |
182 (77) |
|
11 – 15 years |
22 (19) |
20 (17) |
42 (18) |
|
16 – 20 years |
6 (5) |
7 (6) |
13 (5) |
|
Age (years) |
|||
|
Mean |
6.5 |
6.2 |
6.4 |
|
Median |
5 |
4.5 |
4.8 |
|
Range |
1 - 20 |
1 - 18 |
1 - 20 |
|
Ethnicity n (%) |
|||
|
Hispanic |
17 (14) |
23 (19) |
40 (17) |
|
Not Hispanic |
101 (86) |
95 (81) |
196 (83) |
|
Country n (%) |
|||
|
US |
75 (64) |
71 (60) |
148 (62) |
|
Canada |
43 (36) |
48 (40) |
91 (38) |
Clinical Trial Data
Table 6. Demographics of the Efficacy Population
|
Demographic Characteristics |
Trial 1 |
Trial 2 |
Total |
|---|---|---|---|
|
Sex n (%) |
|||
|
Male |
53 (48) |
9 (60) |
62 (50) |
|
Female |
58 (52) |
4 (40) |
62 (50) |
|
Race n (%) |
|||
|
White |
93 (84) |
9 (69) |
102 (82) |
|
Black or African American |
5 (5) |
0 (0) |
5 (4) |
|
Asian |
4 (4) |
2 (15) |
6 (5) |
|
Native Hawaiian or Other Pacific Islander |
2 (1) |
0 (0) |
2 (2) |
|
Other |
7(6) |
2 (15) |
9 (7) |
|
Age Group n (%) |
|||
|
1 – 10 years |
45 (41) |
10 (77) |
55 (44) |
|
11 – 17 years |
57 (51) |
3 (23) |
60 (48) |
|
18 – 26 years |
9 (8) |
0 (0) |
9 (7) |
|
Age (years) |
|||
|
Mean |
10.7 |
6.5 |
8.3 |
|
Median |
12 |
4.5 |
8.3 |
|
Range |
1 - 26 |
1 - 15 |
1 - 26 |
|
Ethnicity n (%) |
|||
|
Hispanic |
24 (22) |
2 (15) |
26 (21) |
|
Not Hispanic |
87 (78) |
11 (85) |
98 (79) |
|
Country n (%) |
|||
|
US |
111 (100) |
13 (100) |
124 (100) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of ASPARLAS were evaluated in two clinical trials.
Trial 1 enrolled patients 1 to 20 years old who were recently diagnosed with ALL or lymphoblastic lymphoma. Patients received ASPARLAS or pegaspargase (a drug that helps convert asparagine to other substances) as part of a combination of drugs used for treatment of ALL. The trial had 2 phases. Patients received ASPARLAS or pegaspagase intravenously on Day 7 of the first phase. Patients received either ASPARLAS intravenously every 3 weeks for 10 doses or pegaspargase intravenously every 2 weeks for 15 doses during the second phase. The benefit of ASPARLAS was assessed by measuring the activity level of asparginase in the blood on Days 7, 11, 18, 25, and 32 of the first phase and comparing it to pegaspargase.
Trial 2 enrolled patients 1 to 18 years old who were recently diagnosed with high-risk ALL. Patients received one of 2 doses of ASPARLAS or pegaspargase intravenously as part of a combination of drugs used for treatment. Patients received ASPARLAs or pegaspargase on Day 4 of the first phase and Days 2 and 22 of the second phase. Patients received up to 12 doses of ASPARLAS or pegaspargase intravenously depending on the bone marrow response. The benefit of ASPARLAS was assessed by measuring the concentration of drug in the body over 25 days (AUC) and the maximum amount of drug in the blood after a single dose (Cmax). The AUC and Cmax of ASPARLAS were compared to pegaspargase.
How were the trials designed?
The efficacy and safety of ASPARLAS were primarily evaluated in two open-label, randomized active-comparator clinical trials.
Trial 1 enrolled patients 1 to 20 years old who were recently diagnosed with ALL or lymphoblastic lymphoma. Patients were randomized to receive ASPARLAS 2500 U/m2 or pegaspargase 2500 U/m2 Intravenously as part of a Dana Farber Cancer Institute (DFCI) Consortium backbone therapy. Patients received ASPARLAS or pegaspargase on Day 7 of the induction phase of treatment. Patients received ASPARLAS every 3 weeks for 10 doses or pegaspargase every 2 weeks for 15 doses during the consolidation phase. The primary efficacy endpoint was the achievement and maintenance of nadir serum asparaginase activity (NSAA) above the level of 0.1 U/mL
Trial 2 enrolled patients 1 to 18 years old who were recently diagnosed with high-risk B-precursor ALL. Patients were randomized to receive one of 2 doses of ASPARLAS (2100 U/M2, 2500 U/M2, or pegaspargase 2500 U/M2, intravenously as a component of an augmented Berlin Frankfurt Münster (BFM) therapy. Patients received ASPARLAS or pegaspargase on Day 4 of the induction phase of treatment. Patients received up to 12 doses of ASPARLAS or pegaspargase depending on the bone marrow response during the induction phase. The benefit of ASPARLAS was assessed by measuring the concentration of drug in the body over 25 days (AUC) and the maximum amount of drug in the blood after a single dose (Cmax). The primary efficacy endpoint was the maximum concentration of drug (Cmax) and the area under the plasma drug concentration-time curve (AUC).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.