Drug Trials Snapshots: CRESEMBA (mucormycosis)
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CRESEMBA Prescribing Information for complete information.
CRESEMBA (isavuconazonium sulfate)
(Crē-SEM-bah)
Astellas Pharma US, Inc.
Approval date: March 6, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CRESEMBA is a drug used to treat adults with a rare but serious fungal infection called invasive mucormycosis. This infection occurs most often in people with weak immune systems.
CRESEMBA is also used to treat adults with a rare but serious fungal infection called invasive aspergillosis. This is discussed in a separate Snapshot.
How is this drug used?
CRESEMBA is administered by a health care professional directly into the bloodstream though a needle in the vein. This is known as an intravenous, or IV, infusion. CRESEMBA is also available as a capsule to be taken by mouth.
What are the benefits of this drug?
The trial showed that CRESEMBA was safe and effective in treating invasive mucormycosis.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes results from the efficacy trial.
Table 3. All-Cause-Mortality and Overall Response Success in Mucorales Patients
| Primary | Refractory | Intolerant | Total | |
|---|---|---|---|---|
| All-cause Mortality through Day 42 | 7 (33%) | 5 (46%) | 2 (40%) | 14 (38%) |
| Overall Response Success Rate at End-of-Treatment | 6/19a (32%) | 4/11 (36%) | 1/5 (20%) | 11/35a (31%) |
a Two primary mucormycosis patients were not assessed at End-of-Treatment due to ongoing treatment.
Source: CRESEMBA Package Insert, Section 14, Table 10
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The number of patients in the trial was limited. Therefore, differences in response to CRESEMBA between men and women could not be determined.
- Race: The number of patients in the trial was limited. Therefore, differences in response to CRESEMBA among races could not be determined.
- Age: The number of patients in the trial was limited. Therefore, differences in response to CRESEMBA among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The table below summarizes efficacy by subgroup.
Table 4. Subgroup Analysis of Primary Endpoint (All-cause mortality through Day 42)
| Subgroup | CRESEMBA (n=37) |
|
|---|---|---|
| n (%) | Total, N | |
| Overall Response/All patients | 14 (37.8) | 37 |
| Sex | ||
| Male | 13 (43.3) | 30 |
| Female | 1 (14.3) | 7 |
| Age Group | ||
| <17 | 0 | |
| >=17 | 10 (31.3) | 32 |
| >=65 years | 4 (80.0) | 5 |
| >=75 years | 3 | 3 |
| Race | ||
| White | 12 (48.0) | 25 |
| Black or African American | 1 (25.0) | 4 |
| Asian | 1 (12.5) | 8 |
| American Indian or Alaska Native | 0 | |
| Native Hawaiian or Other Pacific Islander | 0 | |
| Other | 0 | |
| Missing | 0 | |
| Ethnicity | ||
| Hispanic or Latino | 0 | 1 |
| Not Hispanic or Latino | 14 (38.9) | 36 |
| Missing | 0 | |
| Region | ||
| United States | 8 (50.0) | 16 |
| Rest of World | 6 (28.6) | 21 |
| Canada | 0 | |
| South America | 0 | 1 |
| Europe | 2 (28.6) | 7 |
| Asia | 4 (30.8) | 13 |
| Africa | 0 | |
Source: Clinical Reviewer
What are the possible side effects?
The most common side effects are nausea, vomiting, diarrhea, headache, abnormal liver blood tests, low potassium levels in the blood, constipation, shortness of breath, cough, and swelling of the arms and legs.
CRESEMBA may also cause serious side effects, including liver problems, reactions to the infusion of the drug, and severe allergic and skin reactions.
What are the possible side effects results of trials used to assess safety)?
Table 5. Treatment-Emergent Adverse Reactions in the Clinical Trial with an Incidence of at least 10% in CRESEMBA-treated Patients
| Adverse Event | CRESEMBA (N=257) n (%) |
Voriconazole (N=259) n (%) |
|---|---|---|
| Nausea | 71 (27.6) | 78 (30.1) |
| Vomiting | 64 (24.9) | 73 (28.2) |
| Diarrhea | 61 (23.7) | 60 (23.2) |
| Hypokalemia | 49 (19.1) | 58 (22.4) |
| Elevated liver laboratory tests | 44 (17.1) | 63 (24.3) |
| Dyspnea | 44 (17.1) | 35 (13.5) |
| Abdominal pain | 43 (16.7) | 59 (22.8) |
| Headache | 43 (16.7) | 38 (14.7) |
| Peripheral edema | 39 (15.2) | 47 (17.8) |
| Constipation | 36 (14) | 54 (20.8) |
| Insomnia | 27 (10.5) | 25 (9.7) |
| Fatigue | 27 (10.5) | 18 (6.9) |
| Back pain | 26 (10.1) | 19 (7.3) |
| Renal failure | 26 (10.1) | 21 (8.1) |
Source: Extracted from CRESEMBA Package Insert, Section 6, Table 2
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of overall side effects was similar in men and women.
- Race: The number of non-White, non-Asian patients was limited. The risk of overall side effects was similar in Whites and Asians.
- Age: The risk of overall side effects was similar in patients 65 years and below and those above 65 years. Certain side effects—called serious adverse events1—were seen more frequently in patients above 65 years.
1 Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
Tables 6, 7, and 8 summarize treatment-emergent adverse events (TEAEs) by age, sex, and race in the safety trial.
Table 6. Incidence of TEAEs by Age (Safety Population)
| ≤65 years of age | >65 years of age | |||
|---|---|---|---|---|
| CRESEMBA (N=201) n (%) |
Voriconazole (N=201) n (%) |
CRESEMBA (N=56) n (%) |
Voriconazole (N=58) n (%) |
|
| All TEAEs | 192 (95.5) | 197 (98) | 55 (98.2) | 58 (100) |
| Serious TEAEs | 99 (49.3) | 116 (57.7) | 35 (62.5) | 33 (56.9) |
| TEAEs Leading to Discontinuation of Study Drug | 29 (14.4) | 44 (21.9) | 8 (14.3) | 15 (25.9) |
| TEAEs Leading to Death | 47 (23.4) | 55 (27.4) | 15 (26.8) | 17 (29.3) |
Source: Clinical Review, Table 82
Table 7. Incidence of TEAEs by Sex (Safety Population)
| Males | Females | |||
|---|---|---|---|---|
| CRESEMBA (N=145) n (%) |
Voriconazole (N=163) n (%) |
CRESEMBA (N=112) n (%) |
Voriconazole (N=96) n (%) |
|
| All TEAEs | 139 (95.9) | 161 (98.8) | 108 (96.4) | 94 (97.9) |
| Serious TEAEs | 75 (51.7) | 93 (57.1) | 59 (52.7) | 56 (58.3) |
| TEAEs Leading to Discontinuation of Study Drug | 19 (13.1) | 43 (26.4) | 18 (16.1) | 16 (16.7) |
| TEAEs Leading to Death | 30 (20.7) | 44 (27) | 32 (28.6) | 28 (29.2) |
Source: Clinical Review, Table 83
Table 8. Incidence of TEAEs by Race (Safety Population)
| White | Asian | |||
|---|---|---|---|---|
| CRESEMBA N=211 n (%) |
Voriconazole N=191 n (%) |
CRESEMBA N=44 n (%) |
Voriconazole N=65 n (%) |
|
| All TEAEs | 204 (96.7) | 189 (98.6) | 41 (93.2) | 63 (96.9) |
| Serious TEAEs | 110 (52.1) | 112 (58.6) | 22 (50) | 34 (52.3) |
| TEAEs Leading to Discontinuation of Study Drug | 27 (12.8) | 47 (24.6) | 10 (22.7) | 12 (18.5) |
| TEAEs Leading to Death | 45 (21.3) | 57 (29.8) | 17 (38.6) | 13 (20) |
Source: Clinical Review, Table 84
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA evaluated the benefit of CRESEMBA for the treatment of invasive mucormycosis based on one trial involving 37 patients. The study was conducted in 34 centers in North America, South America, Europe, and Asia.
A second trial, which included 257 patients given CRESEMBA and 259 patients given another medicine for the treatment of a serious fungal infection, provided information about the side effect profile of CRESEMBA in these patients. This trial was conducted in 102 centers in North America, South America, Europe, Asia, and Africa.
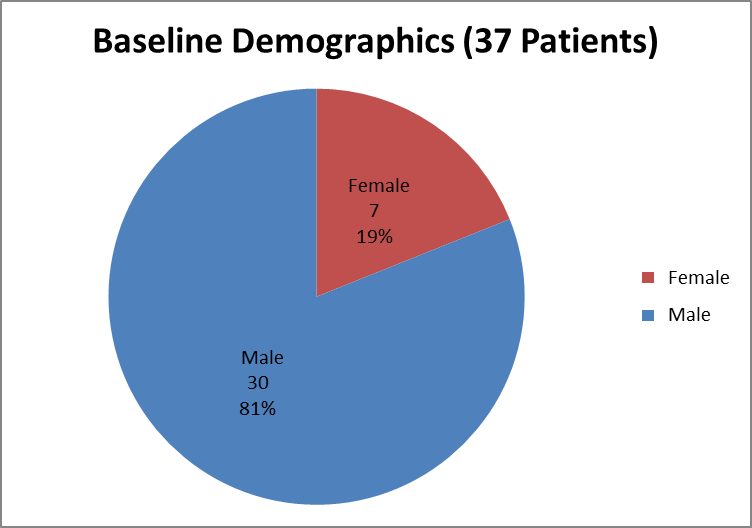
Figure 1 summarizes how many men and women were in the clinical trial used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex (Efficacy)
Source: Clinical Reviewer
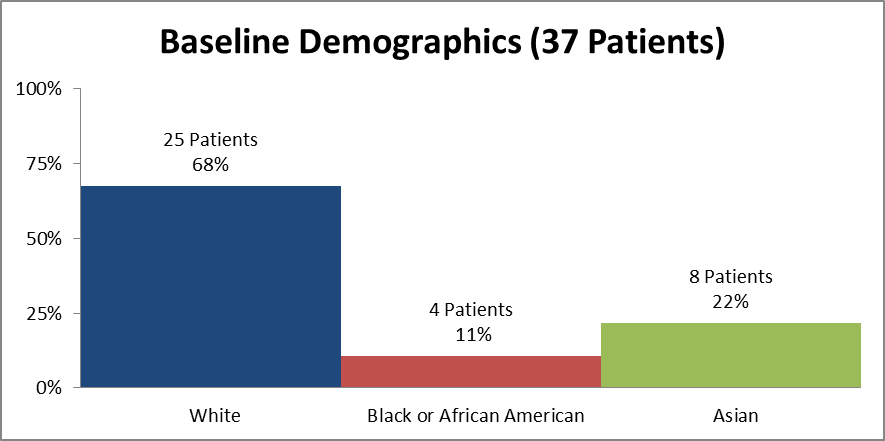
Figure 2 and Table 1 summarize how many patients by race were in the clinical trial used to evaluate efficacy.
Figure 2. Baseline Demographics by Race (Efficacy)
Source: Clinical Reviewer
Table 1. Demographics of Efficacy Trials by Race (Efficacy)
| Race | Number of Patients | Percentage (%) |
|---|---|---|
| White | 25 | 68 |
| Black or African American | 4 | 11 |
| Asian | 8 | 22 |
Source: Clinical Reviewer
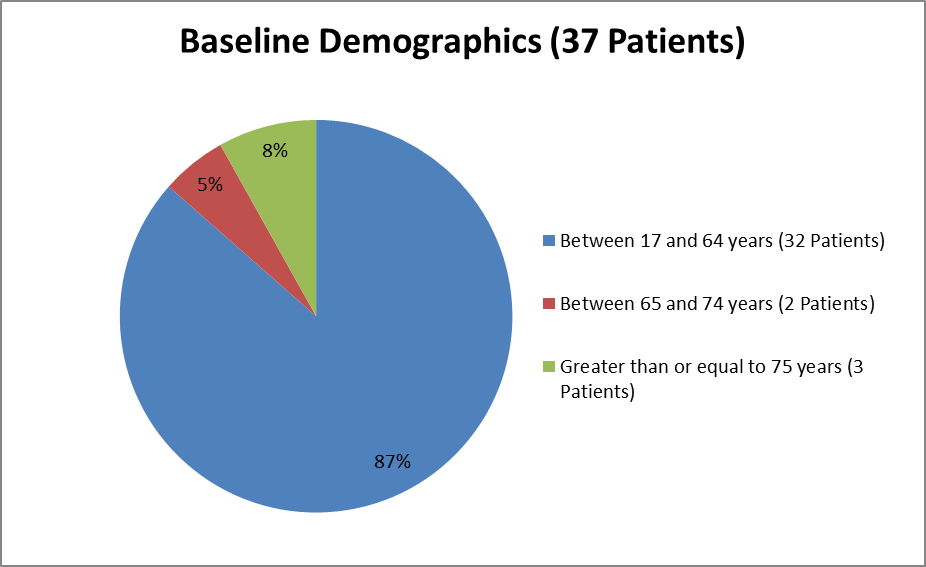
Figure 3 summarizes the ages of patients in the clinical trial used to evaluate efficacy.
Figure 3. Baseline Demographics by Age (Efficacy)
Source: Clinical Reviewer
Who participated in the trials?
The tables below summarize baseline demographics for the efficacy trial and safety trial.
Table 9. Baseline Demographics for the Efficacy Trial
| Demographic Parameters | CRESEMBA (N=37) n (%) |
|---|---|
| Sex | |
| Male | 30 (81.1) |
| Female | 7 (18.9) |
| Age (years) | |
| Mean (SD) | 48.5 (15.5) |
| Median | 50 |
| Min, Max | 22, 79 |
| Age Group | |
| <17> | 0 |
| >=17 - <65> | 32 (86.5) |
| >=65 years | 5 (13.5) |
| >=75 years | 3 (8.1) |
| Race | |
| White | 25 (67.6) |
| Black or African American | 4 (10.8) |
| Asian | 8 (21.6) |
| American Indian or Alaska Native | 0 |
| Native Hawaiian or Other Pacific Islander | 0 |
| Other | 0 |
| Missing | 0 |
| Ethnicity | |
| Hispanic or Latino | 1 (2.7) |
| Not Hispanic or Latino | 36 (97.3) |
| Missing | 0 |
| Region | |
| United States | 16 (43.2) |
| Rest of World | 21 (56.8) |
| Canada | 0 |
| South America | 1 (2.7) |
| Europe | 7 (18.9) |
| Asia | 13 (35.1) |
| Africa | 0 |
Source: Clinical Reviewer
Table 10. Baseline Demographics for the Safety Trial
| Demographic Parameter | CRESEMBA (N=257) n (%) |
Voriconazole (N=259) n (%) |
|---|---|---|
| Sex | ||
| Male | 145 (56.2) | 163 (63.2) |
| Female | 112 (43.8) | 96 (36.8) |
| Age (years) | ||
| Mean (SD) | 51.1 (16.2) | 51.1 (15.8) |
| Median | 54 | 53 |
| Min, Max | 17, 82 | 18, 87 |
| Age Group (years) | ||
| 17 – 64 | 195 (75.6) | 196 (76.0) |
| 65 and older | 63 (24.4) | 62 (24.0) |
| Race | ||
| White | 211 (81.8) | 191 (74.3) |
| Black or African American | 1 (0.4) | 1 (0.4) |
| Asian | 45 (17.4) | 64 (24.9) |
| American Indian or Alaskan Native | 0 | 0 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 |
| Other | 1 (0.4) | 1 (0.4) |
| Missing | 0 | 1 (0.4) |
| Ethnicity | ||
| Hispanic or Latino | 22 (8.5) | 9 (3.5) |
| Not Hispanic or Latino | 236 (91.5) | 248 (96.5) |
| Missing | 0 | 1 (0.4) |
| Region | ||
| United States | 29 (11.2) | 25 (9.7) |
| Rest of World | 229 (88.8) | 233 (90.3) |
| Canada | 1 (0.4) | 3 (1.2) |
| South America | 17 (6.6) | 14 (5.4) |
| Europe | 115 (44.6) | 117 (45.4) |
| Asia | 78 (30.2) | 92 (35.7) |
| Africa | 10 (3.9) | 3 (1.2) |
| Mexico | 2 (0.8) | 1 (1.4) |
| Australia/New Zealand | 6 (2.3) | 3 (1.2) |
Source: Clinical Reviewer
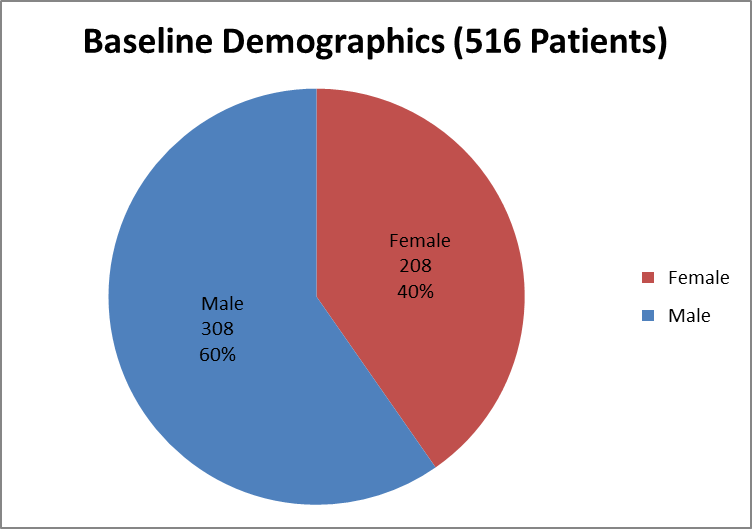
Figure 4 summarizes how many men and women were enrolled in the clinical trial used to evaluate safety.
Figure 4. Baseline Demographics by Sex (Safety)
Source: Clinical Reviewer
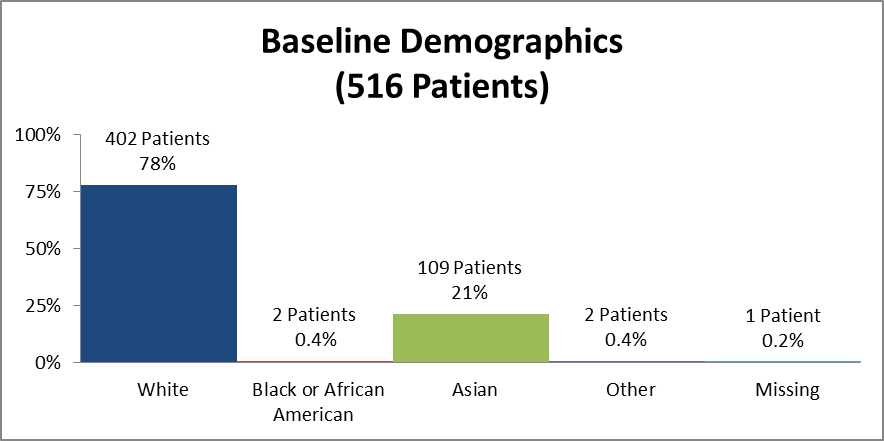
Figure 5 and Table 2 summarize how many patients by race were in the clinical trial used to evaluate safety.
Figure 5. Baseline Demographics by Race (Safety)
Source: Clinical Reviewer
Table 2. Baseline Demographics by Race (Safety)
| Race | Number of Patients | Percentage (%) |
|---|---|---|
| White | 402 | 78 |
| Black or African American | 2 | 0.4 |
| Asian | 109 | 21 |
| Other | 2 | 0.4 |
| Missing | 1 | 0.2 |
Figure 6 summarizes the ages of patients in the clinical trial used to evaluate safety.
Figure 6. Baseline Demographics by Age (Safety)
Source: From Clinical Reviewer
How were the trials designed?
FDA approved CRESEMBA for the treatment of invasive mucormycosis based on two trials. The first trial, which assessed the benefit of CRESEMBA, enrolled 37 patients with invasive mucormycosis and treated them all with CRESEMBA. The trial measured how many patients died during the first 42 days of treatment. The second trial, which included 257 patients given CRESEMBA and 259 patients given another medicine for the treatment of a serious fungal infection, assessed the safety of CRESEMBA in these patients.
How were the trials designed?
For the indication of treatment of invasive mucormycosis, the primary evidence to support the efficacy of CRESEMBA was based on a single, open-label Phase 3 trial. All-cause mortality through Day 42 was measured. This was compared to an appropriate historical control group.
The safety of CRESEMBA was supported by a Phase 3 trial. The trial was a randomized, double-blind, non-inferiority, active-controlled trial which evaluated the safety and efficacy of CRESEMBA versus voriconazole for primary treatment of invasive fungal disease caused by Aspergillus species or other filamentous fungi. Eligible patients had proven, probable, or possible invasive fungal infections per European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria. Patients were stratified by history of allogeneic bone marrow transplant, uncontrolled malignancy at baseline, and by geographic region.
Patients randomized to receive CRESEMBA treatment were administered an IV loading dose of CRESEMBA every 8 hours for the first 48 hours. Beginning on day 3, patients received intravenous or oral therapy of CRESEMBA once daily. Patients randomized to receive voriconazole treatment were administered voriconazole intravenously for the first 48 hours. Therapy could then be switched to an oral formulation of voriconazole. The protocol-defined maximum treatment duration was 84 days.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
MEDICAL REVIEW