Drug Trials Snapshots: DEFITELIO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to DEFITELIO Prescribing Information for complete information.
DEFITELIO (defibrotide sodium)
(def-ih-TELL-ee-yo)
Jazz Pharmaceuticals, Inc.
Approval date: March 30, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DEFITELIO is a drug for the treatment of hepatic veno-occlusive disease (VOD). Hepatic veno-occlusive disease is a condition in which some of the veins in the liver become blocked, causing swelling and a decrease in blood flow inside the liver, which may lead to liver damage and death.
DEFITELIO is for patients with VOD who have an additional kidney or lung abnormalities after they receive a stem cell transplant from blood or bone marrow called hematopoietic stem cell transplantation (HSCT).
How is this drug used?
DEFITELIO is given by healthcare provider using a needle placed in a vein (known as intravenous infusion) every 6 hours for at least 21 days. It takes 2 hours to receive one full dose of DEFITELIO.
What are the benefits of this drug?
In the trials, 38 to 45 percent of patients treated with DEFITELIO were alive 100 days after HSCT. The expected survival rates 100 days after HSCT are 21 to 31 percent for patients who received only supportive care or interventions other than DEFITELIO.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results for the clinical trials based on survival at 100 days after a stem cell transplant (Day + 100 after HSCT).
Table 2. Survival Rates at Day +100 post-HSCT for Individual Trials
|
Trial 1 N=102 |
Trial 2 N=75 |
Trial 3 N=351 |
|
|---|---|---|---|
| Percent of patients | 38% | 44% | 45% |
| 95% CI* | 29%, 48% | 33%, 55% | 40%, 51% |
DEFITELIO PRESCRIBING INFORMATION
CI=Confidence Interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Sex: DEFITELIO worked similarly in male and female patients.
Race: The majority of participants in the clinical trials were White. Differences among races could not be determined due to small number of participants in other races.
Age: DEFITELIO worked similarly in patients younger and older than 16 years of age. Differences between patients younger and older than 65 years of age could not be determined because there were very few patients who were 65 years old or older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figure below summarizes efficacy results by subgroup for Trial 1.
Table 3. Day +100 post-HSCT Survival for Select Subgroups in Trial 1
| Subgroup | Alive/n | Rate (95% CI) |
|---|---|---|
| Overall | 39/102 | 38.2 (28.8,48.4) |
| Age group | ||
| ≤16 years | 22/44 | 50.0 (34.6,65.4) |
| > 16 years | 17/58 | 29.3 (18.1,42.7) |
| Race | ||
| Non-White | 6/25 | 24.0 (9.4,45.1) |
| White | 33/77 | 42.9 (31.6,54.6) |
| Sex | ||
| Female | 14/38 | 36.8 (21.8,54.0) |
| Male | 25/64 | 39.1 (27.1,52.1) |
CI=confidence interval
FDA Statistical review
What are the possible side effects?
The most common side effects that occurred after DEFITELIO treatment was started were low blood pressure, diarrhea, vomiting, nausea and nose bleeds.
DEFITELIO may cause serious side effects including bleeding and allergic reactions.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with DEFITELIO independent of causality.
Table 4. Adverse Reactionsa ≥10% or Grade 4-5 Adverse Reactions ≥2%
| DEFITELIO (n=176) |
||
|---|---|---|
| Adverse Reactiona | Any grade | Grade 4-5b |
| Hypotension | 65 (37%) | 12 (7%) |
| Diarrhea | 43 (24%) | 0 |
| Vomiting | 31 (18%) | 0 |
| Nausea | 28 (16%) | 0 |
| Epistaxis | 24 (14%) | 0 |
| Pulmonary alveolar hemorrhage | 15 (9%) | 12 (7%) |
| Gastrointestinal hemorrhage | 15 (9%) | 5 (3%) |
| Sepsis | 12 (7%) | 9 (5%) |
| Graft versus host disease | 11 (6%) | 7 (4%) |
| Lung infiltration | 10 (6%) | 5 (3%) |
| Pneumonia | 9 (5%) | 5 (3%) |
| Pulmonary hemorrhage | 7 (4%) | 4 (2%) |
| Infection | 6 (3%) | 4 (2%) |
| Hemorrhage intracranial | 5 (3%) | 4 (2%) |
| Hyperuricemia | 4 (2%) | 4 (2%) |
| Cerebral hemorrhagec | 3 (2%) | 3 (2%) |
Were there any differences in side effects among sex, race and age?
Sex: The occurrence of side effects was similar in male and female patients.
Race: The majority of participants in the clinical trials were White. Differences in side effects among races could not be determined due to small number of participants in other races.
Age: The occurrence of side effect was similar in patients younger and older than 16 years of age. Differences in side effects between patients younger and older than 65 years of age could not be determined because there were very few patients who were 65 years old or older.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events by subgroup that occurred in patients after initiation of DEFITELIO treatment.
Table 5. Subgroup Analysis of TEAE*s
| Subgroup of Patients with ≥ 1 TEAE | DEFITELIO (N=176) n/N (%) |
|---|---|
| Sex | |
| Male | 101/105 (96%) |
| Female | 68/71 (96%) |
| Age Group | |
| ≤16 years | 61/65 (94%) |
| >16 years | 108/111(97%) |
| Race | |
| White | 132/137 (96%) |
| Non-White | 37/39 (95%) |
*TEAE=treatment-emergent adverse event defined as any adverse event starting after initiation of DEFITELIO treatment
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved DEFITELIO based on the on evidence from three clinical trials of 528 patients with hepatic veno-occlusive disease. The trials were conducted in the USA, Canada, and Israel.
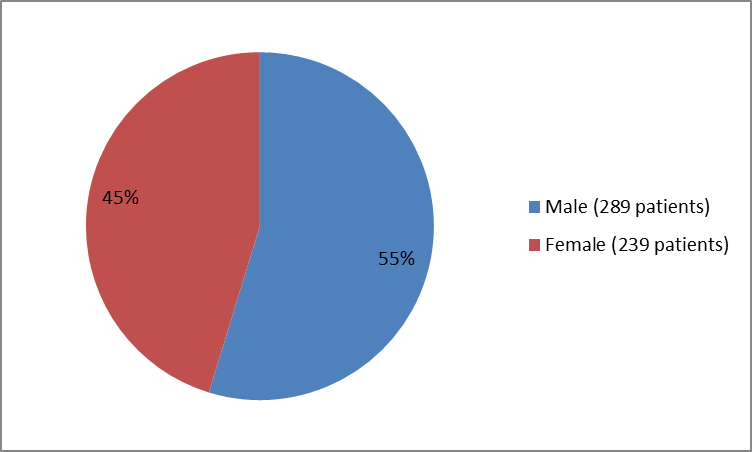
The figure below summarizes how many male and female patients were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
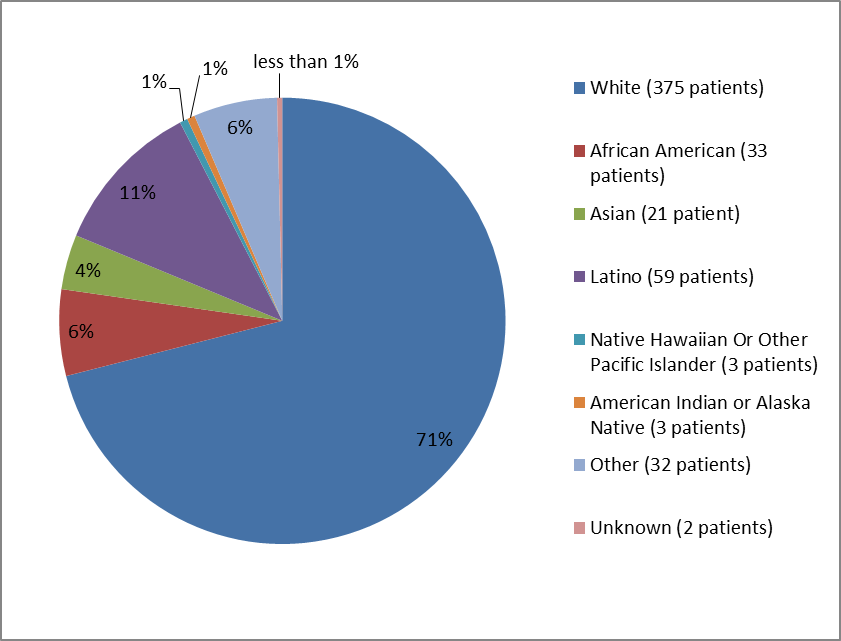
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race*
*Latino ethnicity was included as race
Clinical trial data
Table 1. Baseline Demographics by Race*
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 375 | 71% |
| Black or African American | 33 | 6% |
| Asian | 21 | 4% |
| American Indian or Alaska Native | 3 | 1% |
| Native Hawaiian or Other Pacific Islander | 3 | 1% |
| Other | 32 | 6% |
| Latino | 59 | 11% |
| Unknown | 2 | less than 1% |
*Latino ethnicity was included as race
Clinical trial data
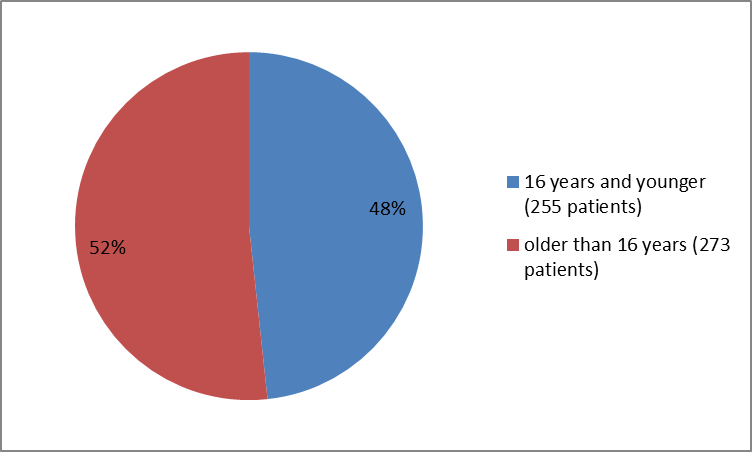
Figure 3 summarizes the percentage of patients by age group in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes baseline demographics of patients in the clinical trials.
Table 6. Baseline Demographics of Patients in the Clinical Trials (efficacy population)
| Characteristic; n (%) | DEFITELIO | |||
|---|---|---|---|---|
| Trial 1 (n=102) |
Trial 2 (n=75) |
Trial 3 (n=351) |
Total (n=528) |
|
| Sex | ||||
| Male | 64 (63) | 41 (55.47) | 184 (52) | 289 (55) |
| Female | 38 (37) | 34 (45.3) | 167 (48) | 239 (45) |
| Race | ||||
| White | 77 (76) | 61 (81) | 237 (68) | 375 (71) |
| African American | 6 (6%) | 6 (8%) | 21 (6%) | 33 (6) |
| Asian | 4 (4%) | 2 (3%) | 15 (4%) | 21 (4) |
| Native Hawaiian Or Other Pacific Islander | 1 (1%) | 0 (0) | 2 (1) | 3 (1) |
| American Indian or Alaska Native | 0 | 1 (1) | 2 (1) | 3 (1) |
| Latino* | 10 (10%) | 4 (5) | 45 (13) | 59 (11) |
| Other | 4 (4%) | 1(%) | 27 (8) | 32 (6) |
| Unknown | 0 | 0 | 2 (1) | 2 (<> |
| Age (years) | ||||
| Mean (SD) | 26.0 (21.37) | 29.3 (18.26) | 21.1 (19.64) | 23.2 (20.01) |
| Median (min, max) | 21.0 (0, 72) | 32.0 (0.4, 61) | 15.0 (0.1, 69) | 17.0 (0, 72) |
| Age group | ||||
| ≤ 16 years | 44 (43) | 22 (29) | 189 (54) | 255 (48) |
| >16 years | 59 (54) | 53 (71) | 162 (46) | 273 (52) |
*Latino ethnicity was included as race
Clinical Trial Data
How were the trials designed?
The benefit and side effects of DEFITELIO were evaluated in three clinical trials of 528 patients with veno-occlusive disease (VOD). All patients also had kidney or lung abnormalities after they received a stem cell transplant from blood or bone marrow called hematopoietic stem cell transplantation (HSCT). All patients received treatment with DEFITELIO.
The benefit of DEFITELIO was evaluated by measuring the proportion of patients who were alive at 100 days after transplantation.
How were the trials designed?
The safety and efficacy of DEFITELIO were established in 3 open-label trials (trials 1 and 2 were prospective trials and trial 3 was an expanded access).
Enrolled patients had hepatic VOD with pulmonary and/or renal dysfunction following hematopoietic stem-cell transplantation (HSCT) and received DEFITELIO for median of 21 days (range 1-83 days).
The efficacy outcome measure was based on survival at 100 days after HSCT (Day + 100).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.