Drug Trials Snapshots: DETECTNET
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DETECTNET Prescribing Information for complete information.
DETECTNET (copper Cu 64 dotatate)
dɪˈtektˌnet
RadioMedix, Inc.
Approval date: September 3, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DETECTNET is a drug for detection of the specific type of tumors called somatostatin receptor positive neuro-endocrine tumors (NETs) in adults.
NETs are rare tumors that develop in certain hormone-producing cells of the body’s neuro-endocrine system.
How is this drug used?
DETECTNET is injected into a vein in preparation for an imaging test (called positron emission tomography scan or PET scan) to help detect the tumor.
What are the benefits of this drug?
enter text
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 shows the performance of DETECTNET in the detection of NETs for Trial 1.
Table 1. Performance of DETECTNET in the Detection of NET by Reader - Trial 1
| NET status as identified by reader | Reference | ||
|---|---|---|---|
| Positive | Negative | ||
| Reader 1 (n=62)* |
Positive | 30 | 1 |
| Negative | 3 | 28 | |
| Percent Reader Agreement (95% CI)** |
91 (75, 98) | 97 (80, 99) | |
| Reader 2 (n=63) |
Positive | 30 | 6 |
| Negative | 3 | 24 | |
| Percent Reader Agreement (95% CI)** |
91 (75, 98) | 80 (61, 92) | |
| Reader 3 (n=63) |
Positive | 30 | 3 |
| Negative | 3 | 27 | |
| Percent Reader Agreement (95% CI)** |
91 (75, 98) | 90 (72, 97) | |
n: number of patients, CI: confidence interval, *Reader 1 interpreted one of the 63 PET scans as “not evaluable”, **Wilson score interval with continuity correction
DETECTNET Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DETECTNET worked similarly in men and women.
- Race: The majority of patients were White. The number of patients of other races were limited; therefore, differences in how the drug worked among races could not be determined.
- Age: DETECTNET worked similarly in patients younger and older than 60 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup efficacy results from Trial 1 are presented below.
Table 2. Efficacy results by Sex-Trial 1
| Reader | Women (N=35) | Men (N=28) | |||
|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | Estimate | 95% CI | |
| Reader 1 | Sensitivitya | 0.857 | (0.6001, 0.959) | 0.947 | (0.754, 0.991) |
| Specificityb | 0.950 | (0.764, 0.991) | 1.000 | (0.701, 1.000) | |
| Reader 2 | Sensitivitya | 0.786 | (0.524, 0.924) | 1.000 | (0.832, 1.000) |
| Specificityb | 0.762 | (0.549, 0.894) | 0.889 | (0.56, 0.9801) | |
| Reader 3 | Sensitivitya | 0.857 | (0.601, 0.959) | 0.947 | (0.754, 0.991) |
| Specificityb | 0.952 | (0.773, 0.992) | 0.778 | (0.453, 0.937) | |
Table 3. Efficacy results by Race-Trial 1
| Reader | White (N=54) | All Other (N=9) | |||
|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | Estimate | 95% CI | |
| Reader 1 | Sensitivity | 0.925 | (0.766, 0.979) | 0.833 | (0.436, 0.969) |
| Specificity | 0.962 | (0.811, 0.993) | 1.000 | (0.439, 1.000) | |
| Reader 2 | Sensitivity | 0.963 | (0.817, 0.993) | 0.667 | (0.300, 0.903) |
| Specificity | 0.778 | (0.592, 0.894) | 1.000 | (0.438, 1.000) | |
| Reader 3 | Sensitivity | 0.926 | (0.766, 0.979) | 0.833 | (0.436, 0.969) |
| Specificity | 0.926 | (0.766, 0.979) | 0.667 | (0.208, 0.936) | |
Table 4. Efficacy results by Age-Trial 1
| Reader | <60 years (N=40) | 60-75 years (N=15) | >75 years (N=8) | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Reader 1 | Sensitivity | 0.929 | (0.68, 0.9873) | 0.929 | (0.685, 0.987) | 0.800 | (0.376, 0.964) |
| Specificity | 0.960 | (0.805, 0.993) | 1.000 | (0.207, 1.000) | 1.000 | (0.444, 1.000 | |
| Reader 2 | Sensitivity | 1.000 | (0.785, 1.000) | 0.857 | (0.601, 0.960) | 0.800 | (0.376, 0.964) |
| Specificity | 0.808 | (0.621, 0.915) | 1.000 | (0.207, 1.000) | 0.667 | (0.208, 0.939) | |
| Reader 3 | Sensitivity | 0.929 | (0.685, 0.987) | 0.929 | (0.685, 0.987) | 0.800 | (0.376, 0.964) |
| Specificity | 0.885 | (0.710, 0.960) | 1.000 | (0.207, 1.000) | 1.000 | (0.439, 1.000 | |
CI=confidence interval
Clinical Trial Report
What are the possible side effects?
DETECTNET is a radioactive drug which may increase the risk of lifetime radiation exposure.
The most common side effects of DETECTNET are nausea, vomiting, and flushing.
What are the possible side effects?
The summary of adverse reactions from the trials is presented below.
In safety and efficacy trials, 71 participants received a single dose of DETECTNET. Of these 71 participants, 21 were healthy volunteers and the remainder were patients with known or suspected NET.
The following adverse reactions occurred at a rate of < 2%:
- Gastrointestinal Disorders: nausea, vomiting
- Vascular Disorders: flushing
In published clinical experience, 126 patients with known history of NET received a single dose of Cu 64 dotatate injection. Four patients were reported to have experienced transient nausea immediately after injection.
DETECTNET Prescribing Information
Were there any differences in side effects among sex, race and age?
Because the side effects were rare, it was not possible to determine the differences in side effects among sex, race and age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The occurrence of the most common adverse reactions per subgroups is presented below.
Table 5. The Most Common Adverse Reactions by Subgroup-Trial 1
| Adverse Reaction | Sex | Race | Age | ||||
|---|---|---|---|---|---|---|---|
| Women N=35 n (%) |
Men N=28 n (%) |
White N=54 n (%) |
All Other N=9 n (%) |
<60 N=40 n (%) |
60-75 N=15 n (%) |
≥75 N=8 n (%) |
|
| Nausea | 0 | 1 (3.6) | 1 (1.9) | 0 | 1 (2.5) | 0 | 0 |
| Vomiting | 1 (2.9) | 1 (3.6) | 2 (3.7) | 0 | 1 (2.5) | 0 | 1 (12.5) |
| Flushing | 0 | 1 (3.6) | 1 (1.9) | 0 | 0 | 1 (6.7) | 0 |
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved DETECTNET based on data from two trials that evaluated 175 adult patients.
Trial 1 evaluated adult patients, some of whom had known or suspected NETs and some of whom were healthy volunteers. The trial was conducted at one site in the United States (Houston, TX).
Trial 2 data came from the literature-reported trial of 112 adult patients, all of whom had history of NETs. The trial was conducted at one site in Denmark.
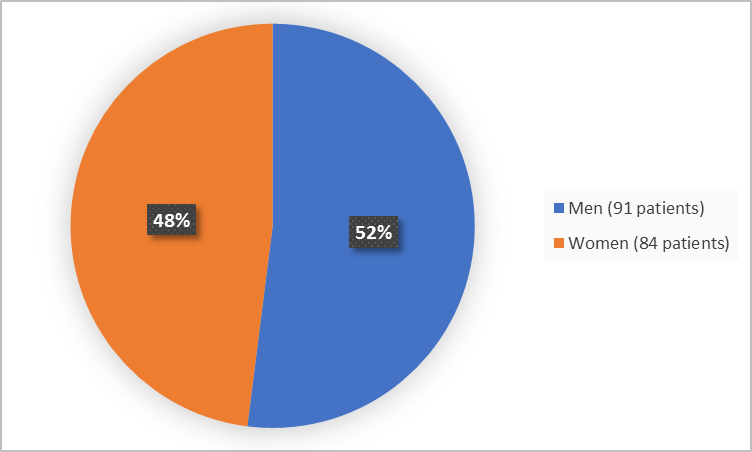
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex
Adapted from FDA Review
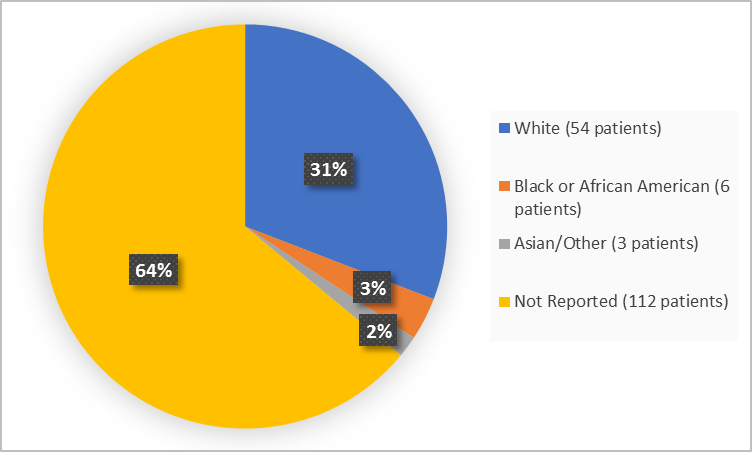
The figure below summarizes patients in the clinical trials by race.
Figure 2. Demographics by Race
Adapted from FDA Review
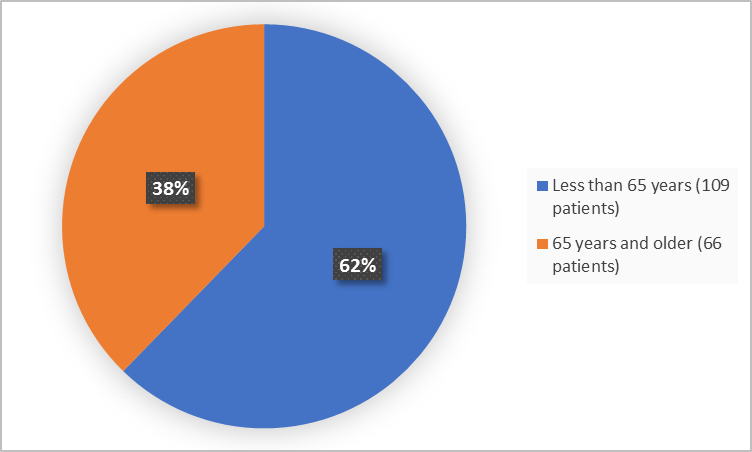
The figure below summarizes patients in the clinical trials by age.
Figure 3. Demographics by Age
Adapted from FDA Review
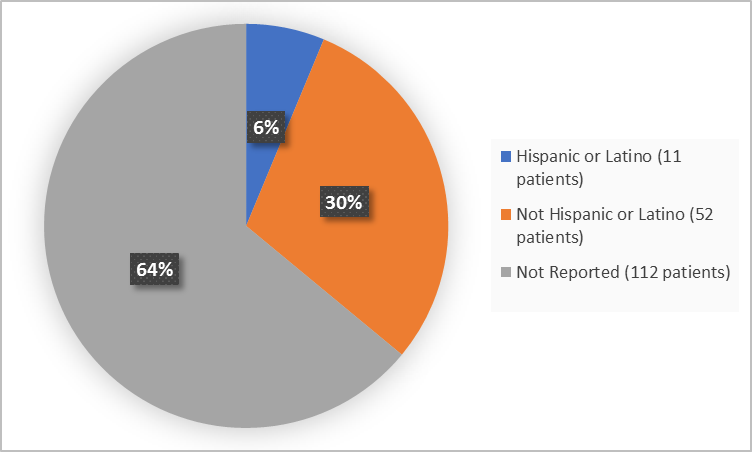
The figure below summarizes patients in the clinical trials by ethnicity.
Figure 3. Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
Table 6. Demographics
| Demographic Characteristics |
Trial 1 N=63 n (%) |
Trial 2 N=112 n (%) |
Total N=175 n (%) |
|---|---|---|---|
| Sex | |||
| Women | 35 (55.6) | 49 (43.8) | 84 (48) |
| Men | 28 (44.4 | 63 (56.3) | 91 (52) |
| Race | |||
| White | 54 (85.7) | 0 | 54 (30.9) |
| Black or African American | 6 (9.5) | 0 | 6 (3.4) |
| Asian | 2 (3.2) | 0 | 2 (1.1) |
| Other | 1 (1.6) | 0 | 1 (0.6) |
| Not Reported | 0 | 112 | 112 (64) |
| Age | |||
| Median | 54 | 63 | - |
| Min, max (years) | 25, 82 | 30, 84 | 25, 84 |
| Age Group | |||
| <65 years | 45 (71.4) | 64 (57.1) | 109 (62.3) |
| ≥65 years | 18 (28.6) | 48 (42.9) | 66 (37.7) |
| Ethnicity | |||
| Hispanic or Latino | 11 (17.5) | 0 | 11 (6.3) |
| Not Hispanic or Latino | 52 (82.5) | 0 | 52 (29.7) |
| Not Reported | 0 | 112 | 112 (64) |
| Region | |||
| USA | 63 (100) | 0 | 63 (36) |
| Denmark | 0 | 112 (100) | 112 (64) |
Adapted from FDA Review
How were the trials designed?
For DETECTNET approval, the FDA evaluated the data from two trials.
In Trial 1, some patients had NETs, and some did not. Both groups received DETECTNET and underwent PET scan imaging.
In Trial 2, all patients had history of NETs and underwent PET scan imaging with DETECTNET.
In both trials, DETECTNET images were compared to either biopsy results or other images taken by different techniques to detect the sites of a tumor. The images were read as either positive or negative for presence of NETs by three independent image readers who did not know patient clinical information.
How were the trials designed?
Trial 1 was a single-center trial that enrolled patients with known or suspected neuroendocrine tumors (NETs) and healthy volunteers. PET imaging results were compared to a composite reference standard consisting of a single oncologist’s blinded assessment of subject diagnosis based on available histopathology results, reports of conventional imaging (MRI, contrast CT, bone scintigraphy, F 18 fludeoxyglucose PET/CT, F 18 sodium fluoride PET/CT, In 111 pentetreotide SPECT/CT, Ga 68 dotatate (PET/CT) performed within 8 weeks prior to DETECTNET imaging, and clinical and laboratory data including chromogranin A and serotonin levels.
DETECTNET images from each patient were interpreted as either positive or negative for NET by three independent readers who were blinded to clinical information and other imaging results.
Trial 2 re-analyzed data from a published single-center trial that enrolled patients with history of neuroendocrine tumors (NETs) and compared copper Cu 64 dotatate imaging to results from other imaging and biopsy.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION