Drug Trials Snapshots: ENSPRYNG
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ENSPRYNG Package Insert for complete information.
ENSPRYNG (satralizumab-mwge)
en-spryng
Genentech, Inc.
Approval date: August 14, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ENSPRYNG is used to treat neuromyelitis optica spectrum disorder (NMOSD) in adult patients with a particular antibody (who are anti-aquaporin-4 or AQP4 antibody positive).
NMOSD is a rare disease that most commonly affects the optic nerves and spinal cord. It is characterized by relapses (attacks) that cause vision loss and serious disability which can lead to death.
How is this drug used?
ENSPRYNG is an injection given under the skin (subcutaneously). The first three injections are given two weeks apart followed by one injection every four weeks.
What are the benefits of this drug?
ENSPRYNG reduces attacks of the disease.
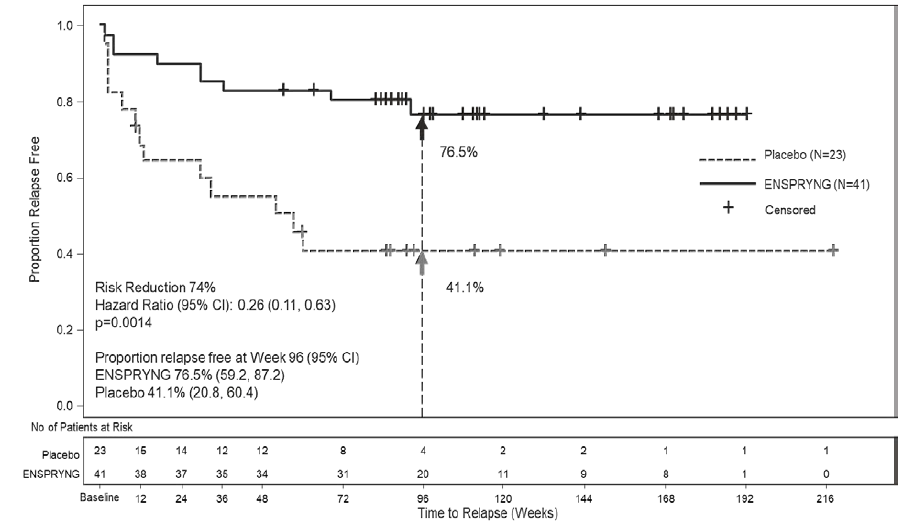
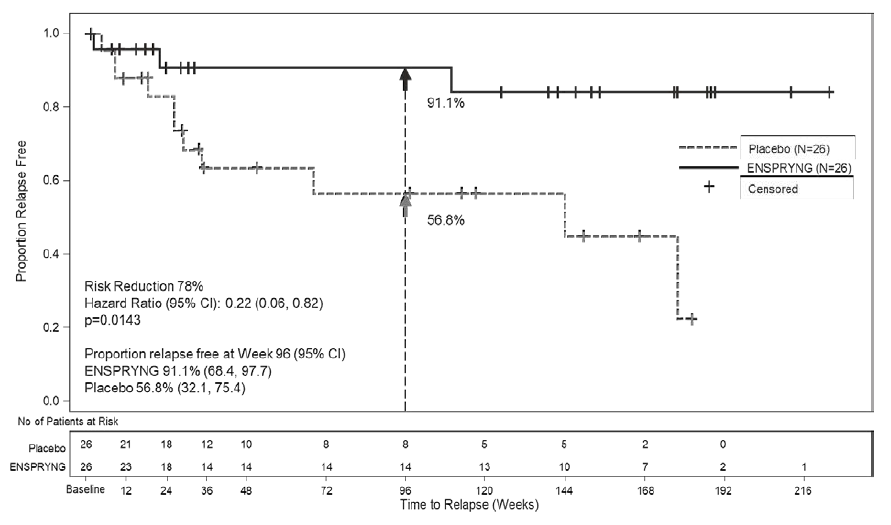
In Trial 1, 76% of ENSPRYNG-treated patients were relapse-free at 96 weeks, compared to 41% placebo-treated patients. In Trial 2, 91% of ENSPRYNG - treated patients were relapse-free at 96 weeks, compared to 57% placebo-treated patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy endpoint for ENSPRYNG was the time to the first adjudicated relapse by blinded Clinical Endpoint Committee (CEC) who determined whether the attack met protocol-defined criteria.
Table 1. Efficacy Results from Trial 1 and Trial 2 in anti-AQP4 Antibody Positive NMOSD Patients
|
|
Trial 1 |
Trial 2 |
||
|
|
ENSPRYNG |
Placebo |
ENSPRYNG |
Placebo |
|
Time to Clinical Endpoint Committee (CEC)-Determined Relapse (Primary Efficacy Endpoint) |
||||
|
Number (%) of Patients with Relapse |
9 (22) |
13 (56.5) |
3 (11.5) |
11 (42.3) |
|
Hazard Ratio |
0.26 |
0.22 |
||
|
p-value |
0.0014 |
0.0143 |
||
|
Risk Reduction |
74% |
78% |
||
|
Proportion of Protocol Defined Relapse-Free Patients at 96 Weeks |
76.5% |
41.1% |
91.1% |
56.8% |
IST=immunosuppressant therapy
Figure 5. Time to First CEC-Determined NMOSD Relapse in the Randomized Controlled Period in the ITT Population Anti-AQP4 Antibody Positive Patients
Figure 6. Time to First CEC-Determined NMOSD Relapse in the Randomized Controlled Period in the ITT Population Anti-AQP4 Antibody Positive Patients
ENSPRYNG Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients in the clinical trials were women. Differences in response to ENSPRYNG between men and women could not be determined.
- Race: ENSPRYNG worked similarly in White and Asian patients. Differences among other races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to ENSPRYNG between patients below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup efficacy analyses for race and age based on primary endpoint are presented below. Sex efficacy analyses were not performed because of female predominance.
Table 2. Analyses of Time to NMOSD Attack by Age and Race
|
|
Trial 1 |
Trial 2 |
||
|
ENSPRYNG |
Placebo |
ENSPRYNG |
Placebo |
|
|
Race White |
7 (17.07)
19 (46.34) |
6 (26.09)
13 (56.52) |
14 (53.85)
12 (46.15) |
13 (50.00)
12 (46.15) |
|
Age Group > 42 years |
15 (36.59)
26 (63.41) |
11 (47.83)
12 (52.17) |
11 (42.31)
15 (57.69) |
10 (38.46)
16 (61.54) |
PDR=protocol defined relapse
Adapted from FDA Statistical Review
What are the possible side effects?
ENSPRYNG may cause serious side effects including infections, increased liver enzymes and decreased neutrophils (type of white blood cells) counts.
The most common side effects are common cold, headache, upper respiratory tract infections, gastritis, rash, joint pain, extremity pain, tiredness and nausea.
What are the possible side effects (results of trials used to assess safety)?
The tables below list adverse reactions from Trials 1 and 2 that occurred in patients treated with ENSPRYNG and at greater incidence than in patients treated with placebo.
Table 3. Adverse Reactions in 4 or More Patients Treated with ENSPRYNG and Greater Incidence than Placebo in Trial 1
|
Adverse Reaction |
ENSPRYNG |
PLACEBO |
|
Rash |
17 |
0 |
|
Arthralgia |
17 |
0 |
|
Pain in extremity |
15 |
9 |
|
Fatigue |
15 |
4 |
|
Nausea |
15 |
9 |
|
Nasopharyngitis |
12 |
4 |
|
Pruritus |
10 |
0 |
|
Depression |
10 |
0 |
|
Cellulitis |
10 |
0 |
|
Neutropenia |
10 |
4 |
|
Blood creatine phosphokinase increased |
10 |
4 |
|
Fall |
10 |
4 |
Table 4. Adverse Reactions Occurring in 3 or More Patients Treated with ENSPRYNG and Greater Incidence than Placebo in Trial 2
|
Adverse Reaction |
ENSPRYNG |
PLACEBO |
|
Nasopharyngitis |
31 |
15 |
|
Headache |
27 |
12 |
|
Upper respiratory tract infection |
19 |
12 |
|
Gastritis |
15 |
0 |
|
Arthralgia |
12 |
0 |
|
Pharyngitis |
12 |
8 |
ENSPRYNG Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The majority patients in the clinical trials were women. Differences in side effects between men and women could not be determined.
- Race: The occurrence of side effects was similar in White and Asian patients. Differences in side effects among other races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Presented below is the analysis of adverse events (AEs) by race from the pooled safety population. The analyses by sex and age were not conducted because of the limited number of male and elderly participants.
Table 5. Adverse Events per Race (pooled safety population)
|
|
ENSPRYNG |
Placebo |
||
|
AE per 100 PY |
95% CI |
AE per |
95% CI |
|
|
All AEs |
478.49 |
(448.18, 510.31) |
506.51 |
(463.38, 552.59) |
|
Overall |
478.49 |
(448.18, 510.31) |
506.51 |
(463.38, 552.59) |
|
White |
498.61 |
(458.54, 541.23) |
534.75 |
(475.37, 599.50) |
|
Asian |
395.94 |
(340.36, 458.01) |
449.98 |
(380.18, 528.89) |
|
Black/AA |
581.69 |
(487.38, 688.93) |
561.62 |
(425.37, 727.64) |
|
Serious AE |
|
|
|
|
|
Overall |
14.97 |
(10.02, 21.50) |
17.98 |
(10.66, 28.42) |
|
White |
13.97 |
(7.99, 22.69) |
18.19 |
(8.72, 33.45) |
|
Asian |
10.94 |
(3.55, 25.52) |
12.24 |
(3.34, 31.35) |
|
Black/AA |
34.73 |
(14.99, 68.43) |
19.71 |
(2.39, 71.18) |
PY=patient-years CI=confidence interval
Clinical Trials Report
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved ENSPRYNG based on evidence from two clinical trials (Trial 1/ NCT02073279 and Trial 2/NCT02028884) of 116 patients with NMOSD who were AQP4 antibody positive. The trials were conducted at 62 sites in the Unites States, Canada, Europe and Asia.
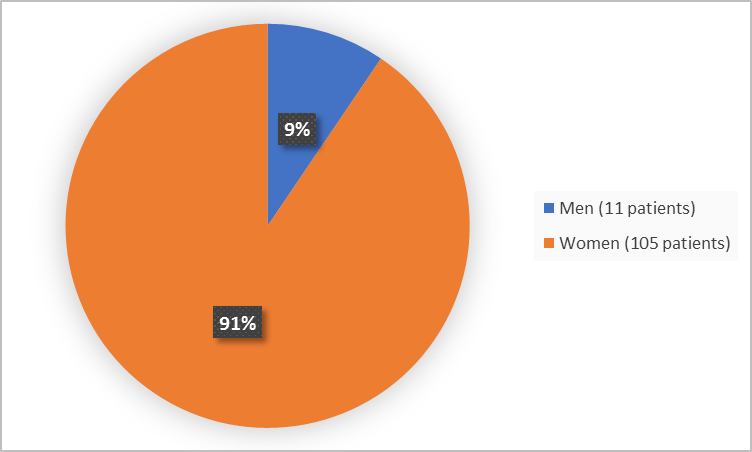
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
FDA Review
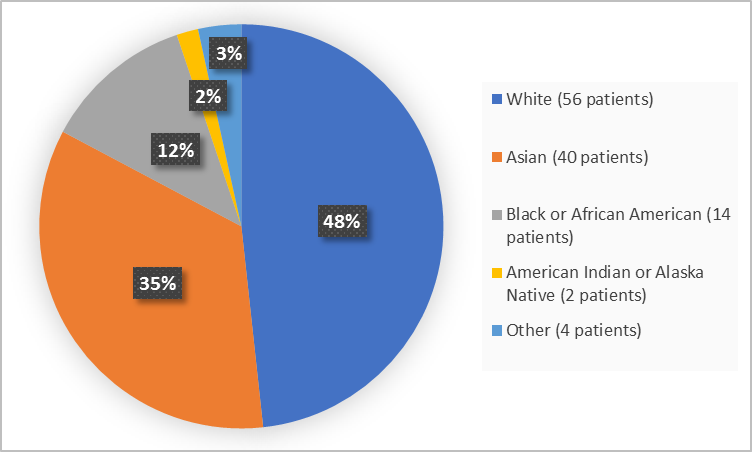
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race
FDA Review
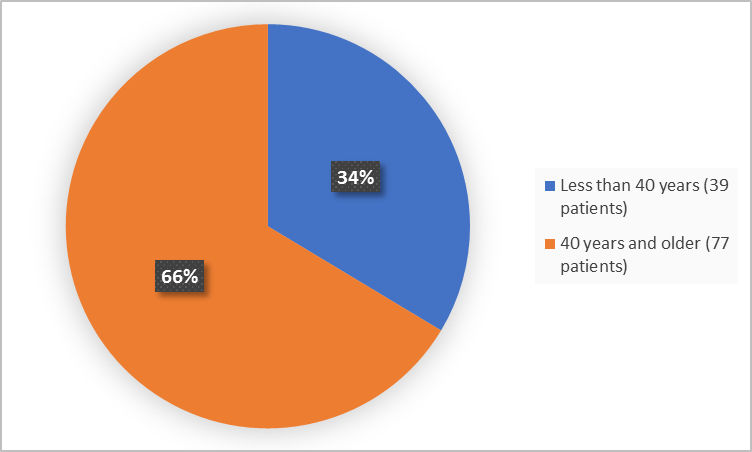
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Demographics by Age
FDA Review
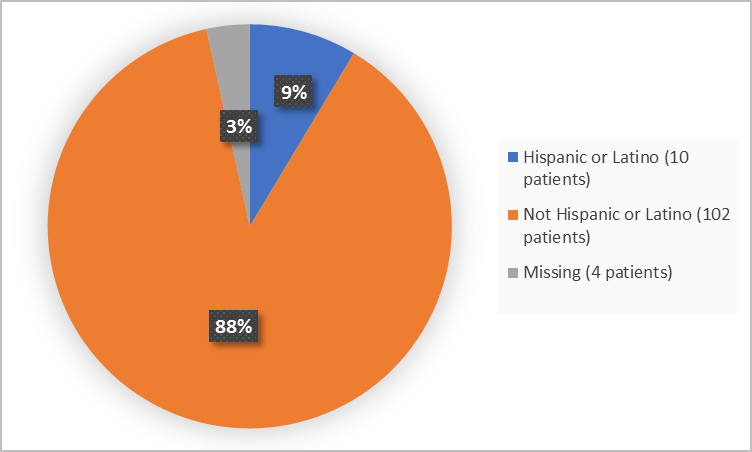
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trial.
Figure 4. Demographics by Ethnicity
FDA Review
Who participated in the trials?
Demographics of the population that participated in the ENSPRYNG trial are presented in the table below.
Table 6. Trial Demographics
|
|
Trial 1 |
Trial 2 |
Total |
|
Sex |
|
|
|
|
Women |
53 (82.8) |
52 (100) |
105 (90.5) |
|
Men |
11 (17.2) |
0 |
11 (9.5) |
|
Race |
|
|
|
|
White |
32 (50.0) |
24 (46.2) |
56 (48.3) |
|
Asian |
13 (20.3) |
27 (51.9) |
40 (34.5) |
|
Black or African American |
14 (21.9) |
0 |
14 (12.1) |
|
American Indian or Alaska Native |
2 (3.1) |
0 |
2 (1.7) |
|
Other |
3 (4.7) |
1 (1.9) |
4 (3.4) |
|
Age |
|
|
|
|
Median |
45 |
45.5 |
45 |
|
Minimum, Maximum |
20,70 |
21,73 |
20, 73 |
|
Age Group |
|
|
|
|
< 40 years |
22 (34.4) |
17 (32.7) |
39 (33.6) |
|
≥ 40 years |
42 (65.6) |
35 (67.3) |
77 (66.4) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
10 (15.6) |
0 |
10 (8.6) |
|
Not Hispanic or Latino |
50 (78.1) |
52 (100) |
102 (87.9) |
|
Missing |
4 (6.2) |
0 |
4 (3.4) |
|
Region |
|
|
|
|
United States |
34 (53.1) |
0 |
34 (29.3) |
|
Europe |
13 (20.3) |
26 (50.0) |
39 (33.6) |
|
Asia |
11 (17.2) |
26 (50.0) |
37 (31.9) |
|
Canada |
6 (9.4) |
0 |
6 (5.2) |
Adapted from FDA Review
How were the trials designed?
There were two trials that evaluated the benefits and side effects of ENSPRYNG. The trials enrolled adult patients with NMOSD who were positive for anti-aquaporin-4 antibody. Patients received at random either ENSPRYNG or placebo injections according to the schedule. Neither the patients nor the healthcare providers knew which treatment was being given. In the second trial, all patients were also receiving their current immunosuppressive medications for the treatment of NMOSD.
The benefit of ENSPRYNG was evaluated by measuring the time to the first attack and comparing it to placebo.
How were the trials designed?
The safety and efficacy of ENSPRYNG were established in two randomized, placebo- controlled trials of patients with NMOSD.
In both trials, patients were randomized to and received ENSPRYNG or placebo. In Trial 2, all patients were also receiving either concurrent azathioprine, oral corticosteroids, or mycophenolate mofetil during the trial.
The primary efficacy endpoint was the time to the onset of the first adjudicated relapse evaluated by a blinded Clinical Endpoint Committee (CEC).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.