Drug Trials Snapshots: HEMLIBRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the HEMLIBRA Package Insert for complete information.
HEMLIBRA (emicizumab)
hem-lee-bruh
Genentech, Inc.
Approval date: November 16, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
HEMLIBRA is a drug used to prevent or reduce frequency of bleeding episodes in children and adult patients with hemophilia A. It is to be used in patients who have developed an immune response known as a FVIII inhibitor or antibody.
Hemophilia A is an inherited blood-clotting disorder that affects primarily males. It is caused by missing or abnormal clotting protein (Factor VIII) making it more difficult to stop bleeding.
How is this drug used?
HEMLIBRA is a preventative (prophylactic) treatment injected under the skin (subcutaneous) once a week. The amount of drug used depends on patient’s weight.
What are the benefits of this drug?
Patients treated with HEMLIBRA experienced fewer bleeding episodes in comparison to patients who received no preventative treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the randomized portion of Trial 1. The primary endpoint was annualized bleed rate (ABR) for treated bleeds.
Table 2. Annualized Bleed Rate with HEMLIBRA Prophylaxis Arm versus No Prophylaxis Arm in Patients ≥ 12 Years of Age
| Endpoint | HEMLIBRA Prophylaxis (N=35) | No Prophylaxis (N=18) |
|---|---|---|
| Treated Bleeds | ||
| ABR (95% CI) a | 2.9 (1.7, 5.0) | 23.3 (12.3, 43.9) |
| % reduction (95% CI), p-value | 87% (72.3%, 94.3%), <> | |
| % patients with 0 bleeds (95% CI) | 62.9 (44.9, 78.5) | 5.6 (0.1, 27.3) |
| Median ABR (IQR) | 0 (0, 3.7) | 18.8 (13.0, 35.1) |
| All Bleeds | ||
| ABR (95% CI)a | 5.5 (3.6, 8.6) | 28.3 (16.8, 47.8) |
| % reduction (95% CI), p-value | 80% (62.5%, 89.8%), <> | |
| % patients with 0 bleeds (95% CI) | 37.1 (21.5, 55.1) | 5.6 (0.1, 27.3) |
| Treated Spontaneous Bleeds | ||
| ABR (95% CI)a | 1.3 (0.7, 2.2) | 16.8 (9.9, 28.3) |
| % reduction (95% CI), p-value | 92% (84.6%, 96.3%), <> | |
| % patients with 0 bleeds (95% CI) | 68.6 (50.7, 83.1) | 11.1 (1.4, 34.7) |
| Treated Joint Bleeds | ||
| ABR (95% CI)a | 0.8 (0.3, 2.2) | 6.7 (2.0, 22.4) |
| % reduction (95% CI), p-value | 89% (48%, 97.5%), 0.0050 | |
| % patients with 0 bleeds (95% CI) | 85.7 (69.7, 95.2) | 50.0 (26.0, 74.0) |
| Treated Target Joint Bleeds | ||
| ABR (95% CI)a | 0.1 (0.03, 0.6) | 3.0 (1.0, 9.1) |
| % reduction (95% CI), p-value | 95% (77.3%, 99.1%), 0.0002 | |
| % patients with 0 bleeds (95% CI) | 94.3 (80.8, 99.3) | 50.0 (26.0, 74.0) |
ABR = annualized bleed rate; CI = confidence interval; IQR = interquartile range, 25th percentile to 75th percentile
a Based on negative binomial regression.
HEMLIBRA Prescribing Information
Table 3. Annualized Bleed Rate with HEMLIBRA Prophylaxis in Pediatric Patients ≥ 12 Years of Age (Interim Analysis)
| Endpoint | ABRa (95% CI) N = 23 |
Median ABR (IQR) N = 23 |
% Zero Bleeds (95% CI) N = 23 |
|---|---|---|---|
| Treated Bleeds | 0.2 (0.1, 0.6) | 0 (0, 0) | 87 (66.4, 97.2) |
| All Bleeds | 2.9 (1.8, 4.9) | 1.5 (0, 4.5) | 34.8 (16.4, 57.3) |
| Treated Spontaneous Bleeds | 0.1 (0, 0.5) | 0 (0, 0) | 95.7 (78.1, 99.9) |
| Treated Joint Bleeds | 0.1 (0, 0.5) | 0 (0, 0) | 95.7 (78.1, 99.9) |
| Treated Target Joint Bleeds | Not Estimable* | 0 (0, 0) | 100 (85.2, 100) |
ABR = annualized bleed rate; CI = confidence interval; IQR = interquartile range, 25th percentile to 75th percentile
* No treated target joint bleeds reported
a Based on negative binomial regression
HEMLIBRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
• Sex: All patients were males; therefore, differences in response among sexes could not be determined.
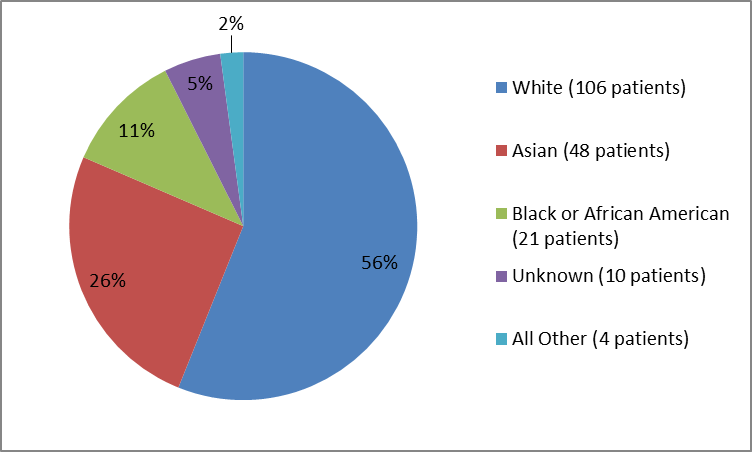
• Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in response among races could not be determined.
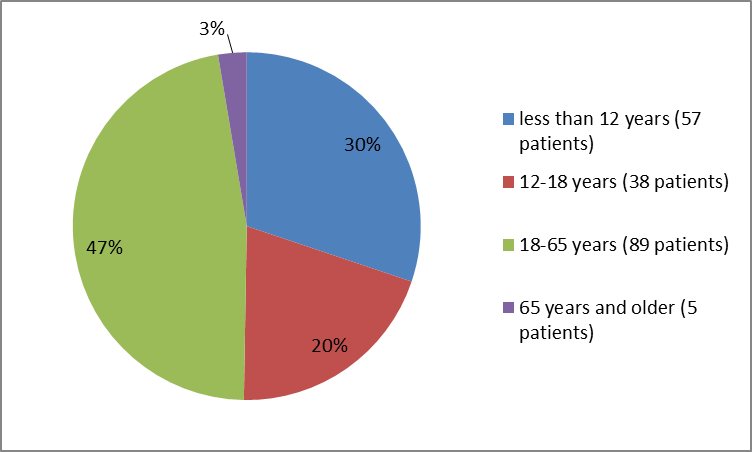
• Age: HEMLIBRA worked similarly in patients younger than and older than 18 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below presents efficacy results for the randomized portion of Trial 1 by age and race subgroups. Analysis by sex was not performed because all patients were males. Because Trial 2 was not randomized, no subgroup analyses were performed.
Table 4. Subgroup Analyses of Annualized Bleed Rate (ABR) for treated bleeds by Age and Race-Trial 1
| HEMLIBRA Prophylaxis (N=35) |
No prophylaxis (N=18) |
ABR ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| # subjects | ABR | # subjects | ABR | |||
| Age (years) | <> | 4 | 0.4 | 2 | 10.9 | 0.04 (0.005, 0.314) |
| ≥ 18 | 31 | 3.6 | 16 | 28.1 | 0.13 (0.055, 0.301) | |
| <> | 34 | 3.2 | 17 | 26.7 | 0.12 (0.051, 0.286) | |
| ≥ 65 | 1 | 3.4 | 1 | 18.3 | 0.18 (0.008, 4.376) | |
| Race | White | 21 | 4.7 | 10 | 31.9 | 0.15 (0.052, 0.407) |
| Asian | 10 | 1.6 | 3 | 36.8 | 0.04 (0.011, 0.164) | |
| Black/African American | 4 | 0.5 | 4 | 7.1 | 0.07 (0.008, 0.600) | |
| Other | 0 | NE | 1 | NE | NE | |
Adapted from FDA Review
What are the possible side effects?
HEMLIBRA may cause severe blood clots called thrombotic microangiopathy and thromboembolism. Thrombotic microangiopathy is a condition that causes blood clots and injury to small blood vessels in organs. Thromboembolism is a condition that causes blood clots to travel and obstruct blood vessels.
The most common side effects of HEMLIBRA are injection site reactions, headache, and joint pain.
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions with frequency of 5% or greater that were observed in the safety population.
Table 5. Adverse Reactions Reported in ≥ 5% of Patients from Pooled Clinical Trials with HEMLIBRA (safety population)
| Body System | Adverse Reaction | Number of Patients n (%) (N=189) |
|---|---|---|
| General Disorders and Administration Site Conditions | Injection site reaction* | 35 (19%) |
| Pyrexia | 13 (7%) | |
| Nervous System Disorders | Headache | 28 (15%) |
| Gastrointestinal Disorders | Diarrhea | 12 (6%) |
| Musculoskeletal and Connective Tissue Disorders | Arthralgia | 18 (10%) |
| Myalgia | 9 (5%) |
* Includes injection site bruising, injection site discomfort, injection site erythema, injection site hematoma, injection site induration, injection site pain, injection site pruritus, injection site rash, injection site reaction, injection site swelling, injection site urticarial, and injection site warmth.
HEMLIBRA Prescribing Information
Were there any differences in side effects among sex, race and age?
• Sex: All patients were male; therefore, differences in side effects among sexes could not be determined.
• Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
• Age: The occurrence of side effects was similar in patients younger and older than 18 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the frequency of the three most common adverse reactions by race and age for the safety population.
Table 6. Pooled Safety Analysis by Race (safety population)
| Adverse Reaction | Race | |||
|---|---|---|---|---|
| White N=106 |
Asian N=48 | Black or African American N=21 |
Other/Unknown N=14 |
|
| Injection site reactions, n (%) | 18 (17%) | 11 (23%) | 1 (5%) | 5 (36%) |
| Headache, n (%) | 15 (14%) | 6 (13%) | 2 (10%) | 5 (36%) |
| Arthralgia, n (%) | 11 (10%) | 2 (4%) | 2 (10%) | 2 (14%) |
Clinical Trial Data
Table 7. Pooled Safety Analysis by Age (safety population)
| Adverse Reaction | Age | ||
|---|---|---|---|
| < 18="" years=""> N =95 |
≥18 years N=94 |
Number of Patients N=189 |
|
| Injection site reactions, n (%) | 18 (19%) | 17 (18%) | 35 (19%) |
| Headache, n (%) | 17 (18%) | 11 (12%) | 28 (15%) |
| Arthralgia, n (%) | 7 (7%) | 11 (12%) | 18 (10%) |
Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved HEMLIBRA based on evidence from three clinical trials, Trial 1 (NCT02622321), Trial 2 (NCT02795767), and Trial 3 of 189 patients with hemophilia A.
Trial 1 was conducted at 43 sites in the following 14 countries: Australia, Costa Rica, France, Germany, Italy, Japan, New Zealand, Poland, South Africa, South Korea, Spain, Taiwan, United Kingdom, and the United States.
Trial 2 was conducted at 24 sites in the following 10 countries: Costa Rica, France, Germany, Italy, Japan, South Africa, Spain, Turkey, United Kingdom, and the United States.
Trial 3 was conducted in Japan.
Figures below summarize 189 patients by sex, race and age who were considered in the assessment of HEMLIBRA’s safety (safety population). This population includes all patients who received at least one dose of the drug any time during the drug development.
Patient demographics by trials are presented under MORE INFO.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race in clinical trials.
Figure 2. Baseline Demographics by Race (safety population)
FDA Review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 106 | 56 |
| Black or African American | 21 | 11 |
| Asian | 48 | 26 |
| American Indian or Alaska Native | 1 | less than 1 |
| Native Hawaiian or Other Pacific Islander | 1 | less than 1 |
| Multiple | 2 | 1 |
| Unknown | 10 | 5 |
Adapted from FDA Review
Figure 3 summarizes the percentage of patients by age in clinical trials.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Who participated in the trials?
The safety population of 189 patients includes any patient who received at least one dose of HEMLIBRA across the clinical development program and is presented below.
Table 8. Demographic Characteristics of Safety Population
| Demographic Parameters | All patients N=189 n (%) |
|---|---|
| Sex | |
| Male | 189 (100%) |
| Age (years) | |
| Median (Min, Max) | 17 (1, 75) |
| Mean (SD) | 24 (17.7) |
| Age Group (years) | |
| <> | 57 (30.2) |
| 12-18 | 38 (20.1) |
| 18-65 | 89 (47.1) |
| ≥65 | 5 (2.6) |
| Race | |
| White | 106 (56.1) |
| Black or African American | 21 (11.1) |
| Asian | 48 (25.4) |
| American Indian or Alaska Native | 1 (0.5) |
| Native Hawaiian or Other Pacific Islander | 1 (0.5) |
| Multiple | 2 (1.1) |
| Unknown | 10 (5.3) |
| Ethnicity | |
| Hispanic or Latino | 23 (12.2) |
| Not Hispanic or Latino | 146 (77.2) |
| Not reported/Unknown | 20 (10.6) |
Clinical Trial Data
The tables below summarize demographics of patients enrolled in Trials 1 and 2.
Table 9. Demographic Characteristics for Trials 1 and 2 (enrolled population)
| Demographic Parameters | Trial 1 (N=109) n (%) |
Trial 2 (N=60) n (%) |
|---|---|---|
| Sex | ||
| Male | 109 (100%) | 60 (100%) |
| Age | ||
| Mean years (SD) | 31.5 (15.8) | 6.9 (3.3) |
| Median (years) | 28.0 | 7 |
| Age Group | ||
| < 65=""> | 105 (96.3%) | 60 (100%) |
| > 65 years | 4 (3.7%) | 0 |
| Race | ||
| White | 71 (65.1%) | 32 (53.3%) |
| Black or African American | 11 (10.1%) | 10 (16.7%) |
| Asian | 21 (19.3%) | 10 (16.7%) |
| Other/Unknown | 6 (5.5%) | 8 (1.3%) |
| Ethnicity | ||
| Hispanic or Latino | 18 (16.5) | 5 (8.3) |
| Not Hispanic or Latino | 91 (83.5) | 53 (88.3%) |
| Unknown | 0 | 2 (3.4) |
| Region | ||
| Africa | 6 (5.5) | 6 (10) |
| Asia | 17 (15.6) | 9 (15%) |
| Australia and Oceania | 6 (5.5) | 0 |
| Central America | 5 (4.6) | 1(1.6) |
| North America | 39 (35.8) | 17 (28.3) |
| Europe | 36 (33.0) | 27(45%) |
Adapted from FDA Review
The table below summarizes demographics of patients enrolled in Trial 3 that provided additional safety data.
Table 10. Demographic Characteristics for Trial 3 (enrolled population in Part C)
| Demographic Parameters | N = 18 n (%) |
|---|---|
| Age | |
| Median (Min, Max) | 30 (12, 58) |
| Mean | 32.9 (15.4) |
| Gender | |
| Male | 18 (100%) |
| Race | |
| Asian | 18 (100%) |
| Country | |
| Japan | 18 (100%) |
Adapted from FDA Review
How were the trials designed?
Trial 1 enrolled patients 12 years and older with hemophilia A with Factor VIII inhibitors. Approximately half of the patients who were not receiving prophylaxis with a bypassing agent prior to enrollment on the study received, in random order, either HEMLIBRA once a week or no preventative treatment for 24 weeks. These patients were evaluated for benefit and side effects.
Trial 2 enrolled patients younger than 12 years of age with hemophilia A with factor VIII inhibitors. All patients received HEMLIBRA once a week. At the time of data analysis (interim analysis) only patients who completed at least 12 weeks of treatment with HEMLIBRA were evaluated for benefit. All enrolled patients were evaluated for side effects.
In Trial 3, patients 12 years and older received HEMLIBRA subcutaneously once a week for longer than 52 weeks. Patients were evaluated primarily for side effects.
The benefit of HEMLIBRA was assessed in Trial 1 and Trial 2 by counting the number of bleeding episodes during treatment.
How were the trials designed?
FDA approved HEMLIBRA based primarily on data from three, open-label, multicenter trials in male patients with hemophilia A with Factor VIII inhibitors.
In Trial 1, patients 12 years and older with hemophilia A with factor VIII inhibitors were enrolled. In the randomized portion of the trial, patients received either HEMLIBRA treatment or no preventative treatment. Patients, who received treatment, received HEMLIBRA subcutaneously at a dose of 3 mg/kg/week for 4 weeks, followed by 1.5 mg/kg weekly for a total of 24 weeks. To obtain additional efficacy and safety data, all patients could continue their HEMLIBRA dose of 1.5 mg/kg/week after completing 24 weeks of treatment, and patients who were randomized to no preventative treatment could start HEMLIBRA treatment after 24 weeks.
In Trial 2, pediatric patients less than 12 years old, could have been either on prophylaxis or episodic treatment with bypassing agents prior to study enrollment. All patients were treated with HEMLIBRA subcutaneously at a dose of 3 mg/kg/week for 4 weeks, followed by 1.5 mg/kg/week. An interim efficacy analysis was conducted on patients who received at least 12 weeks of treatment. Patients who continued to benefit from HEMLIBRA treatment could continue treatment.
The efficacy outcomes, in both trials, were the annualized bleeding rates.
In Trial 3 which was a dose finding trial, patients 12 years and older with hemophilia A received HEMLIBRA at three different dosing regimens. Trial data was provided for safety analysis only.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION