Drug Trials Snapshots: IBSRELA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the IBSRELA Prescribing Information for complete information.
IBSRELA (tenapanor)
(ibs rel`a)
Ardelyx
Approval date: September 12, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
IBSRELA is a treatment for adults with a disease of the gut called irritable bowel syndrome with constipation commonly referred to as IBS-C.
How is this drug used?
IBSRELA is a tablet taken by mouth two times each day.
What are the benefits of this drug?
Higher proportion of patients who received IBSRELA experienced a decrease in abdominal pain and more frequent bowel movements in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for each trial based on daily diary entries. In both trials, the primary endpoint was the proportion of responders. A responder was defined as a patient achieving both the abdominal pain intensity and stool frequency criteria in the same week for at least 6 of the first 12 weeks of treatment.
Table 2. Efficacy Responder Rates in Placebo-Controlled Trials (Trial 1 and Trial 2) in Adults with IBS-C: Responder for at least 6 of the First 12 Weeks of Treatment
| Trial 1 | |||
|---|---|---|---|
| IBSRELA N=293 |
Placebo N=300 |
Treatment Difference [95% CIa] |
|
| Responderb Components of Responder Endpoint: CSBM Responderc Abdominal Pain Responderd |
37% 47% 50% |
24% 33% 38% |
13% [6%, 20%] |
| Trial 2 | |||
| Responder Rates | IBSRELA N=307 |
Placebo N=299 |
Treatment Difference [95% CIa] |
| Responderb Components of Responder Endpoint: CSBM Responderc Abdominal Pain Responderd |
27% 34% 44% |
19% 29% 33% |
8% [2%, 15%] |
a CI: Confidence Interval
bA responder for these trials was defined as a patient who met both the abdominal pain and CSBM weekly responder criteria for at least 6 of the first 12 weeks.
cA CSBM responder was defined as a patient who achieved an increase in at least 1 CSBM per week, from baseline, for a least 6 of at least 12 weeks.
dAn abdominal pain responder was defined as a patient who met the criteria of at least 30% reduction from baseline in weekly average of the worst daily abdominal pain, for at least 6 of the first 12 weeks.
Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients in the trials were women. IBSRELA was similarly effective in men and women.
- Race: IBSRELA was similarly effective in different races.
- Age: IBSRELA was similarly effective in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize the results for the primary endpoint in the trial by subgroup.
Table 3. Subgroup Analysis of Primary Endpoint-Trial 1 (ITT population)
| Subgroup | IBSRELA n/N (%) |
Placebo n/N (%) |
Difference (95% CI) |
|---|---|---|---|
| Sex | |||
| Women | 91/240 (37.9%) | 62/247 (25.1%) | 12.8% (4.6%, 21.0%) |
| Men | 16/53 (30.2%) | 9/53 (17.0%) | 13.2% (-3.8%, 29.2%) |
| Race | |||
| White | 69/185 (37.3%) | 47/192 (24.5%) | 12.8% (3.6%, 22.1%) |
| Black or African American | 35/92 (38.0%) | 22/92 (23.9%) | 14.1% (0.9%, 27.3%) |
| Asian | 1/12 (8.3%) | 2/9 (22.2%) | -13.9% (-45.2%, 17.5%) |
| Age Category | |||
| <65 years | 101/271 (37.3%) | 65/273 (23.8%) | 13.5% (5.8%, 21.1%) |
| ≥65 years | 6/22 (27.3%) | 6/27 (22.2%) | 5.1% (- 19.3%, 29.4%) |
Table 4. Subgroup Analysis of Primary Endpoint-Trial 2 (ITT population)
| Subgroup | IBSRELA n/N (%) |
Placebo n/N (%) |
Difference (95% CI) |
|---|---|---|---|
| Sex | |||
| Women | 88/244 (36.1%) | 79/249 (31.7%) | 4.3% (-4.0%, 12.7%) |
| Men | 16/63 (25.4%) | 9/50 (18.0%) | 7.4% (-7.7%, 22.5%) |
| Race | |||
| White | 55/201 (27.4%) | 28/186 (15.1%) | 12.3% (4.3%, 20.3%) |
| Black or African American | 26/88 (29.6%) | 24/100 (24.0%) | 5.6% (-7.1%, 18.2%) |
| Asian | 0/10 (0%) | 1/4 (25%) | -25% (-67.4%, 17.4%) |
| Age Category | |||
| <65 years | 71/279 (25.5%) | 52/277 (18.8%) | 6.7% (- 0.2%, 13.6%) |
| ≥65 years | 12/28 (42.9%) | 4/22 (18.2%) | 24.7% (0.3%, 49.1%) |
FDA Review
What are the possible side effects?
IBSRELA may cause serious dehydration in children. It should not be given to children younger than 6 years of age and avoided in children 6 to 12 years of age.
The most common side effects of IBSRELA are diarrhea, which sometimes may be severe, abdominal distension, excessive gas and dizziness.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions for the Trial 1.
Table 5. Most Common Adverse Reactions* in Patients with IBS-C in Trial 1 (26 Weeks)
| Adverse Reactions | IBSRELA N=293 % |
Placebo N=300 % |
|---|---|---|
| Diarrhea | 16 | 4 |
| Abdominal Distension | 3 | <1 |
| Flatulence | 3 | 1 |
| Dizziness | 2 | <1 |
* Reported in at least 2% of patients in IBSRELA-treated patients and at an incidence greater than placebo
In Trial 2 (610 patients: 309 IBSRELA-treated and 301 placebo-treated) the most common adverse reactions were diarrhea (15% with IBSRELA vs 2% with placebo) and abdominal distension (2% with IBSRELA vs 0% with placebo).
Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The majority of patients in the trials were women. The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in different races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Per trial analyses of subgroup treatment emergent adverse events (TEAEs) and most common adverse reaction (diarrhea) are presented below.
Table 6. Subgroup Analysis of Patients with Any TEAEs and Diarrhea -Trial 1 (safety population)
| Demographic Subgroup | IBSRELA N=293 |
Placebo N=300 | Any TEAE | Risk Difference % (95% CI) |
Diarrhea | Risk Difference % (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| IBSRELA N=146 n (%) |
Placebo N=109 n (%) |
ISBRELA N=48 n (%) |
Placebo (N=11) n (%) |
|||||
| Sex | ||||||||
| Men | 53 | 53 | 22 (42%) | 1 (32%) | 10 (-8,28) | 7 (13%) | 0 (0%) | 13 (8,18) |
| Women | 240 | 247 | 124 (52%) | 108 (44%) | 8 (-1,17) | 41 (17%) | 11 (4.5%) | 12.5 (7.5,17) |
| Race | ||||||||
| White | 185 | 192 | 92 (50%) | 77 (40%) | 10 (0,20) | 39 (21%) | 8 (4.2%) | 17 (10,24) |
| Black or African American | 92 | 92 | 49 (53%) | 42 (46%) | 7 (-7,21) | 8 (8.7%) | 3 (3.3) | 5.4 (-1.6,12) |
| Asian | 12 | 9 | 3 (25%) | 4 (44%) | -19 (-60,22) | 0 (0%) | 0 (0%) | 0 |
| American Indian or Alaska Native | 1 | 0 | 0 (0%) | 0 (0%) | 0 | 0 (0%) | 0 (0%) | 0 |
| All Other | 3 | 7 | 2 (67%) | 2 (29%) | 38 (-25,101) | 1 (33%) | 0 (0%) | 33 (-20,86) |
| Age | ||||||||
| ≤65 years | 273 | 276 | 136 (50%) | 115(42%) | 8 (0,16) | 46 (17%) | 11 (4.0%) | 13 (8,18) |
| >65 years | 20 | 24 | 10 (50%) | 10 (42%) | 8 (-21,37) | 2 (10%) | 0 (0%) | 10 (-3,23) |
Table 7. Subgroup Analysis of Patients with Any TEAEs and Diarrhea -Trial 2 (safety population)
| Demographic Subgroup | IBSRELA N=309 |
Placebo N=301 |
Any TEAE | Risk Difference % (95% CI) |
Diarrhea | Risk Difference % (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| ISBRELA N=188 n (%) |
Placebo N=94 n (%) |
IBSRELA N=80 n (%) |
Placebo N=5 n (%) |
|||||
| Sex | ||||||||
| Men | 64 | 50 | 24 (38%) | 11 (22%) | 16 (-1,33) | 8 (12%) | 0 (0%) | 12 (4,20) |
| Women | 245 | 251 | 164 (67%) | 83 (33%) | 34 (26,42) | 72 (29%) | 5 (2%) | 27 (21,31) |
| Race | ||||||||
| White | 202 | 188 | 125 (62%) | 54 (29%) | 33 (24,42) | 56 (29%) | 2 (1.1%) | 28 (22,34) |
| Black or African American | 89 | 100 | 53 (60%) | 34 (34%) | 26 (12,40) | 19 (21%) | 2 (2%) | 19 (10,28) |
| Asian | 10 | 4 | 5 (50%) | 2 (50%) | 0 | 1 (10%) | 1 (25%) | -15 (-61,31) |
| American Indian or Alaska Native | 0 | 2 | 0 (0%) | 0 (0%) | 0 | 0 (0%) | 0 (0%) | 0 |
| All Other | 8 | 7 | 5 (62%) | 4 (57%) | 5( -45,55) | 0 (0%) | 0 (0%) | 0 |
| Age | ||||||||
| ≤65 years | 284 | 281 | 175 (62%) | 90 (32%) | 30 (22,38) | 74 (26%) | 5 (1.8%) | 24 (19,29) |
| >65 years | 25 | 20 | 13 (52%) | 4 (20%) | 32 (6,58) | 6 (24%) | 0 (0%) | 24 (7,41) |
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved IBSRELA based primarily on evidence from two clinical trials Trial 1(NCT 02686138) and Trial 2(NCT 02621892) of 1199 patients 18 to 75 years of age with IBS-C. The trials were conducted at 214 sites in the United States.
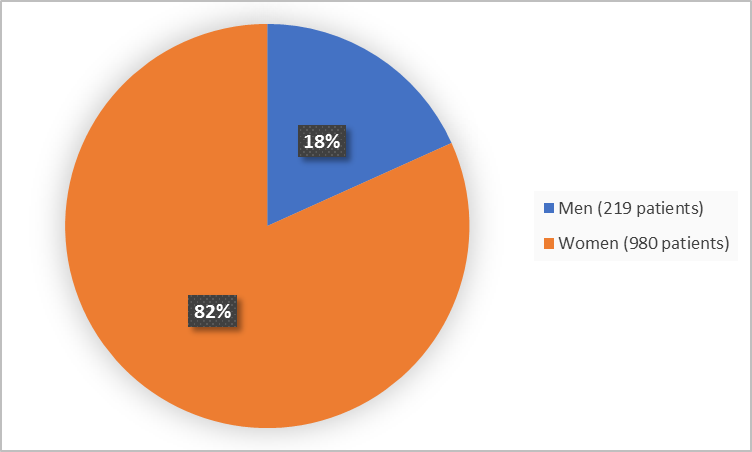
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate the benefit of IBSRELA.
Figure 1. Baseline Demographics by Sex (efficacy population)
Clinical Trial Data
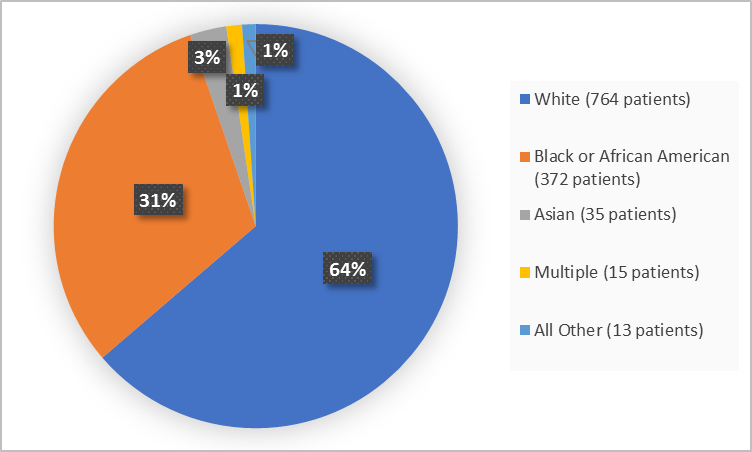
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials used to evaluate the benefit of IBSRELA.
Figure 2. Baseline Demographics by Race (efficacy population)
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race (efficacy population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 764 | 64 |
| Black or African American | 372 | 31 |
| Asian | 35 | 3 |
| American Indian or Alaska Native | 3 | Less than 1 |
| Multiple | 15 | 1 |
| Other/Unknown | 10 | 1 |
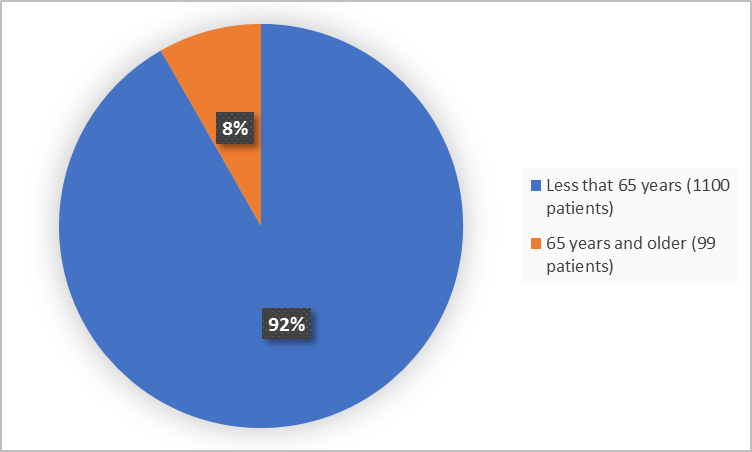
Figure 3. Baseline Demographics by Age (efficacy population)
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics for the efficacy (ITT) population.
Table 8. Demographic of ITT Population
| Demographic Parameters | Trial 1 | Trial 2 | Total (N=1199) n (%) |
||
|---|---|---|---|---|---|
| IBSRELA (N=293) n (%) |
Placebo (N=300) n (%) |
IBSRELA (N=307) n (%) |
Placebo (N=299) n (%) |
||
| Sex | |||||
| Men | 53 (18.1) | 53 (17.7) | 63 (20.5) | 50 (16.7) | 219 (18.3) |
| Women | 240 (81.9) | 247 (82.3) | 244 (79.5) | 249 (83.3) | 980 (81.7) |
| Race | |||||
| White | 185 (63.1) | 192 (64) | 201 (65.5) | 186 (62.2) | 764 (63.7) |
| Black or African American | 92 (31.4) | 92 (30.7) | 88 (28.7) | 100 (33.4) | 372 (31) |
| Asian | 12 (4.1) | 9 (3) | 10 (3.3) | 4 (1.3) | 35 (2.9) |
| American Indian or Alaska Native |
1 (0.3) | 0 | 0 | 2 (0.7) | 3 (0.3) |
| Multiple | 1 (0.3) | 3 (1) | 6 (2) | 5 (1.7) | 15 (1.3) |
| Other/Unknown | 2 (0.6) | 4 (1.3) | 2 (0.7) | 2 (0.7) | 10 (0.8) |
| Age | |||||
| Median (years) | 46 | 44 | 45 | 45 | 45 |
| Min, Max (years) | 18, 74 | 18,75 | 18, 73 | 18,75 | 18,75 |
| Age Category | |||||
| <65 years | 271 (92.5) | 273 (91) | 279 (90.9) | 277 (92.6) | 1100 (91.7) |
| >=65 years | 22 (7.5) | 27 (9) | 28 (9.1) | 22 (7.4) | 99 (8.3) |
| Ethnicity | |||||
| Hispanic or Latino | 77 (26.3) | 78 (26) | 98 (31.9) | 83 (27.8) | 336 (28) |
| Not Hispanic or Latino | 216 (73.7) | 222 (74) | 209 (68.1) | 216 (72.2) | 863 (82) |
| Region | |||||
| United States | 293 (100) | 300 (100) | 299 (100) | 307 (100) | 1199 (100) |
Clinical Trial Data
How were the trials designed?
There were two trials that evaluated the benefit and side effects of IBSRELA. In each trial, patients were randomly assigned to receive either IBSRELA or placebo two times daily for at least 12 weeks. Neither the patients nor the health care providers knew which treatment was being given. After these 12 weeks of treatment, patients in Trial 1 continued same trial design for an additional 14 weeks, and patients in Trial 2 entered a different trial design called Randomized Withdrawal for 4 weeks, where some patients continued to receive IBSRELA, and others were switched to placebo, in a blinded fashion.
In both trials, patients used diaries to record the degree of abdominal pain and stool frequency. The benefit of IBSRELA was assessed by improvements in abdominal pain and complete spontaneous bowel movements in comparison to placebo.
How were the trials designed?
Two randomized, multi-center, double-blind, placebo-controlled trials were conducted to assess the efficacy and safety of IBSRELA in comparison to placebo in adult patients with IBS-C.
The trial designs were identical through the first 12 weeks of treatment, and thereafter differed in that Trial 1 continued for an additional 14 weeks of treatment (26 weeks double-blind treatment), whereas Trial 2 included a 4-week randomized withdrawal (RW) period.
Efficacy of IBSRELA was assessed using responder analyses based on daily diary entries.
In both trials, the primary endpoint was the proportion of responders, where a responder was defined as a patient achieving
- at least a 30% reduction in the weekly average of abdominal pain score compared with baseline, and
- an increase of at least 1 complete spontaneous bowel movement in the same week for at least 6 of the first 12 weeks of treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.