Drug Trials Snapshots: LUCEMYRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the LUCEMYRA Package Insert for complete information.
LUCEMYRA (lofexidine hydrochloride)
LEW-sem-EER-uh
US WorldMeds
Approval date: May 16, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LUCEMYRA is used to lessen symptoms associated with opioid withdrawal during abrupt opioid discontinuation.
How is this drug used?
LUCEMYRA is a tablet taken 4 times daily every 5 – 6 hours for up to 7 days. LUCEMYRA may be continued for up to an additional 7 days based on symptoms.
What are the benefits of this drug?
Patients treated with LUCEMYRA experienced improvement of withdrawal symptoms and completed the preplanned treatment period more frequently in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
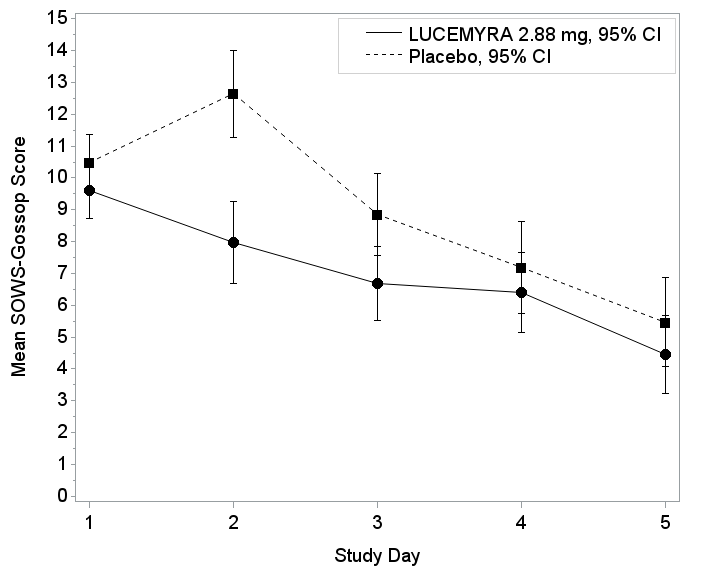
The figures below summarize the mean SOW-Gossop score change results for Trials 1 and 2 based on the intent to treat (ITT) population.
Figure 4. SOWS-Gossop Scores by Study Day in Trial 1
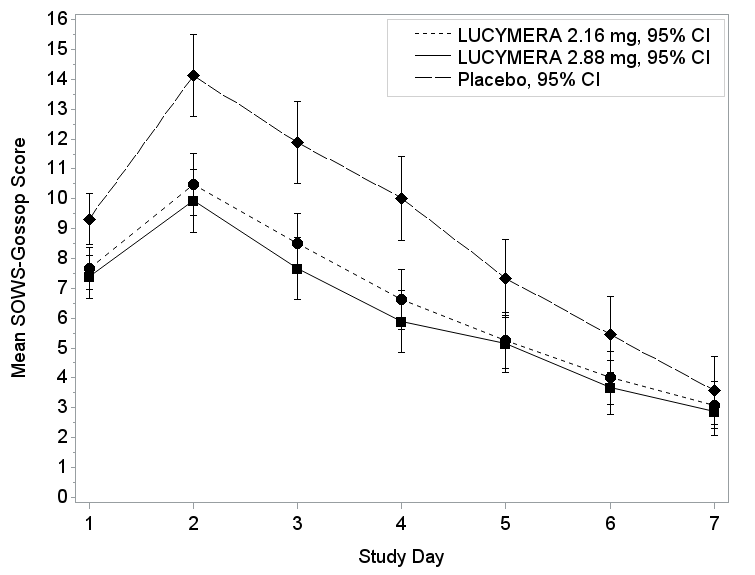
Figure 5. Mean SOWS-Gossop Scores for Days 1 – 5 in Trial 2
LUCEMYRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LUCEMYRA worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
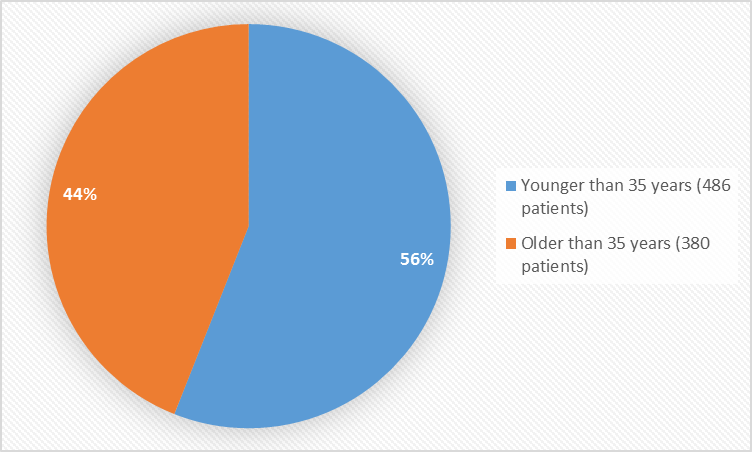
- Age: LUCEMYRA worked similarly in patients younger and older than 35 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex, race, and age. Racial subgroup differences were investigated between White race and all other races combined, because the majority of patients were White. Due to the small sample size, these exploratory analyses should be interpreted with caution.
Figure 6. Subgroup Analysis for Trial 1
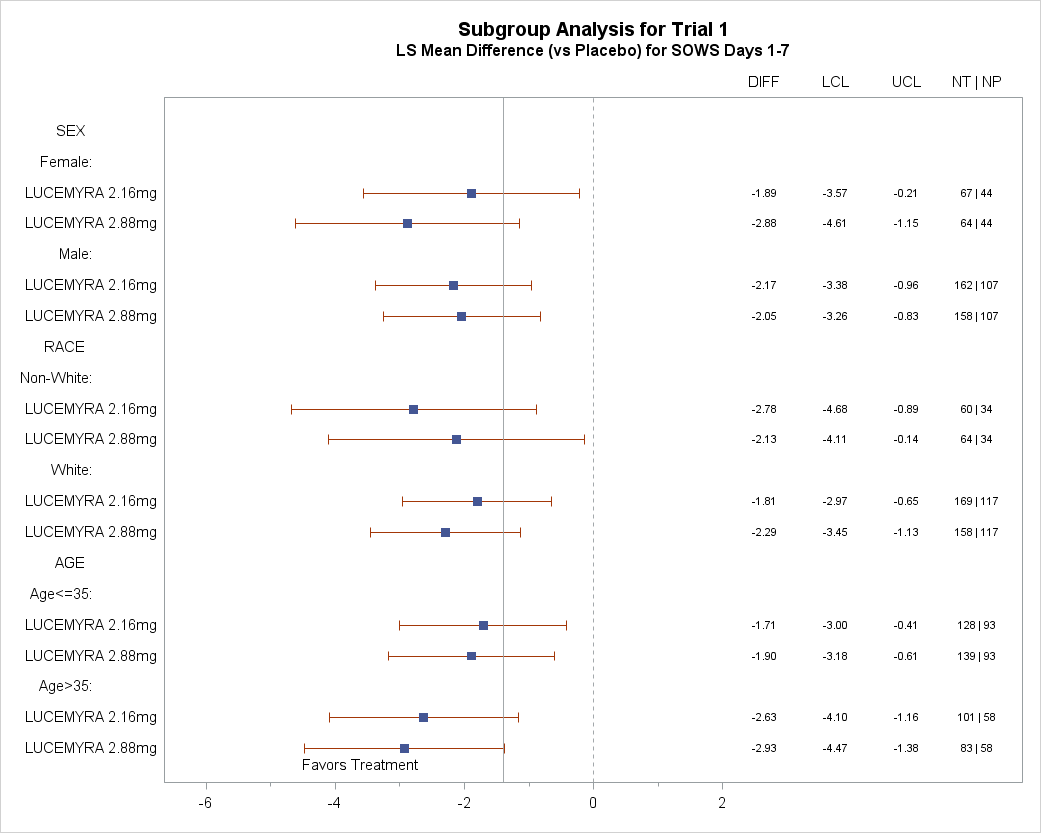
LCL= lower confidence limit
UCL= upper confidence limit
NT = number of patients in the treatment group
NP = number of patients in the placebo group
FDA Review
Figure 7. Subgroup Analysis for Trial 2
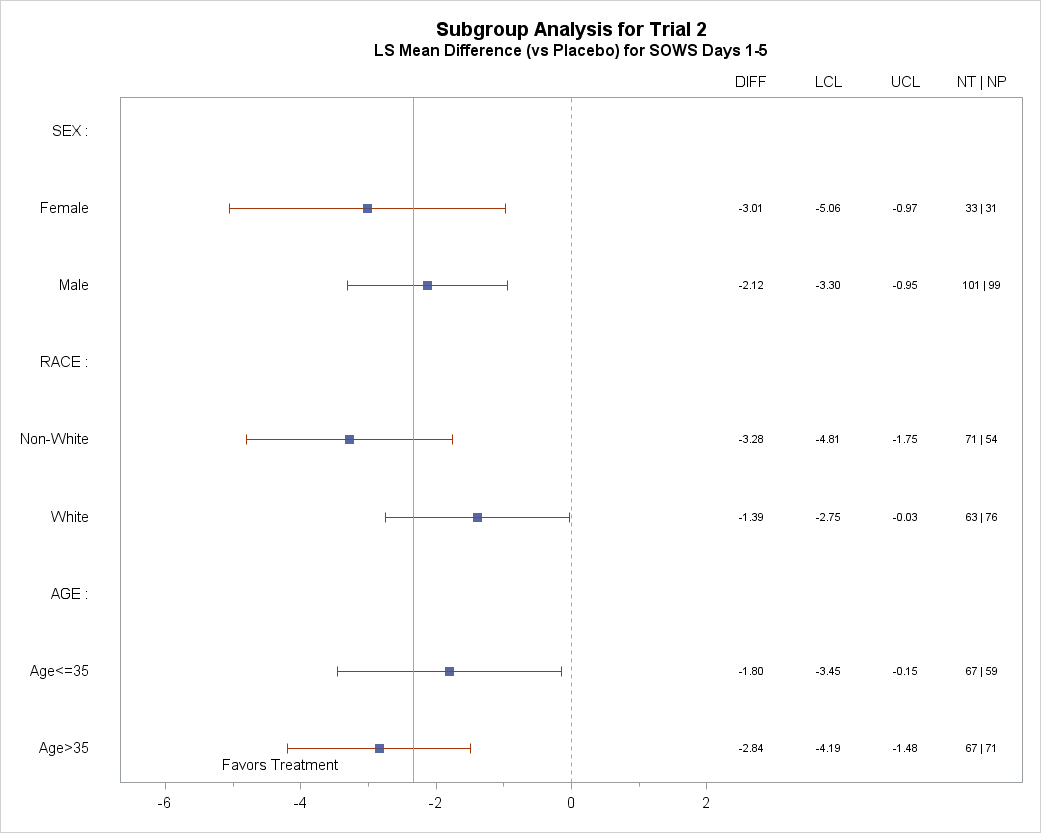
LCL= lower confidence limit
UCL= upper confidence limit
NT = number of patients in the treatment group
NP = number of patients in the placebo group
FDA Review
What are the possible side effects?
LUCEMYRA may cause serious side effects including decreased blood pressure, decreased heart rate, and fainting.
The most common side effects of LUCEMYRA are decreased blood pressure, slow heart rate, dizziness, sleepiness and dry mouth.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in Trial 1.
Table 2: Adverse Reactions Reported by ≥10% of LUCEMYRA-Treated Patients and More Frequently than Placebo (Trial 1)
| Adverse Reaction | LUCEMYRA 2.16 mg1 (%) N=229 |
LUCEMYRA 2.88 mg1 (%) N=222 |
Placebo (%) N=151 |
| Insomnia | 51 | 55 | 48 |
| Orthostatic Hypotension | 29 | 42 | 5 |
| Bradycardia | 24 | 32 | 5 |
| Hypotension | 30 | 30 | 1 |
| Dizziness | 19 | 23 | 3 |
| Somnolence | 11 | 13 | 5 |
| Sedation | 13 | 12 | 5 |
| Dry Mouth | 10 | 11 | 0 |
1 Assigned dose; mean average daily dose received was 79% of assigned dose due to dose-holds for out-of-range vital signs.
LUCEMYRA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: More females that received the highest studied dose of LUCEMYRA (2.88 mg per day) had to stop taking LUCEMYRA, due to slow heart rate and decreased blood pressure.
- Race: The occurrence of side effects between White and Black or African American patients was similar. Because of the small number of patients in other races, it was not possible to determine whether the rates of side effects were different from White or Black patients.
- Age: The occurrence of side effects was similar in patients younger and older than 35 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
As shown in the table below, there was a higher proportion of female patients compared to male patients who had to stop taking or who missed one or more doses of LUCEMYRA because of low heart rate or low blood pressure in the higher dose group.
Table 3: Discontinuations and Dose Holds for Bradycardia and Orthostatic Hypotension by LUCEMYRA Dose and Sex (Trial 1)
| LUCEMYRA 2.16 mg | LUCEMYRA 2.88 mg | |
| Male | 22/162 (14%) | 29/158 (18%) |
| Female | 9/67 (13%) | 20/64 (31%) |
LUCEMYRA Prescribing Information
The tables below summarize the most common adverse reactions that are more notable than placebo (orthostatic hypotension, bradycardia, hypotension, and dizziness,) by subgroup in Trial 1. Due to the small sample size of some subgroups, these exploratory analyses should be interpreted with caution.
Table 4. Subgroup Analysis of Orthostatic Hypotension (Trial 1)
| Demographic Parameters | LUCEMYRA (2.16 mg) n/N (%) |
LUCEMYRA (2.88 mg) n/N (%) |
Placebo n/N (%) |
|---|---|---|---|
| Sex | |||
| Male | 50/162 (30.1%) | 67/158 (42.4%) | 4/107 (3.7%) |
| Female | 18/67 (26.9%) | 34/64 (53.1%) | 4/44 (9.1%) |
| Race | |||
| White | 48/169 (28.4%) | 68/158 (43.0%) | 7/117 (6.0%) |
| Black or African American | 20/54 (37.0%) | 25/48 (52.1%) | 1/26 (3.8%) |
| Other | 0 | 8/16 (50.0%) | 0 |
| Age Group | |||
| 35 years | 39/128 (30.5%) | 58/139 (41.7%) | 5/93 (5.4%) |
| > 35 years | 29/101 (28.7%) | 43/83 (51.8%) | 3/58 (5.2%) |
Clinical Trial Data
Table 5. Subgroup Analysis of Bradycardia (Trial 1)
| Demographic Parameters | LUCEMYRA (2.16 mg) n/N (%) |
LUCEMYRA (2.88 mg) n/N (%) |
Placebo n/N (%) |
|---|---|---|---|
| Sex | |||
|
Male |
37/162 (22.8%) |
49/158 (31.0%) |
6/107 (5.6%) |
|
Female |
18/67 (26.9%) |
21/64 (32.8%) |
3/44 (6.8%) |
| Race | |||
|
White |
43/169 (25.4%) |
61/158 (38.6%) |
8/117 (6.8%) |
|
Black or African American |
11/54 (20.4%) |
14/48 (29.2%) |
0 |
|
Other |
1/16 (6.3%) |
5/16 (31.3%) |
1/8 (12.5%) |
| Age Group | |||
|
35 years |
33/128 (25.8%) |
44/139 (31.7%) |
7/93 (7.5%) |
|
> 35 years |
22/101 (21.8%) |
26/83 (31.3%) |
2/58 (3.4%) |
Clinical Trial Data
Table 6. Subgroup Analysis of Hypotension (Trial 1)
| Demographic Parameters | LUCEMYRA (2.16 mg) n/N (%) |
LUCEMYRA (2.88 mg) n/N (%) |
Placebo n/N (%) |
|---|---|---|---|
| Sex | |||
| Male | 47/162 (29.0%) | 46/158 (29.1%) | 2/107 (1.9%) |
| Female | 24/67 (36.3%) | 23/64 (35.9%) | 1/44 (2.3%) |
| Race | |||
| White | 57/169 (35.8%) | 49/158 (31.0%) | 3/117 (2.6%) |
| Black or African American | 14/54 (25.9%) | 15/48 (31.3%) | 0 |
| Other | 0 | 3/16 (18.8%) | 0 |
| Age Group | |||
| 35 years | 36/128 (28.3%) | 41/139 (29.5%) | 2/93 (2.2%) |
| > 35 years | 35/101 (34.7%) | 28/83 (33.7%) | 1/58 (1.7%) |
Clinical Trial Data
Table 7. Subgroup Analysis of Dizziness (Trial 1)
| Demographic Parameters | LUCEMYRA (2.16 mg) n/N (%) |
LUCEMYRA (2.88 mg) n/N (%) |
Placebo n/N (%) |
|---|---|---|---|
| Sex | |||
| Male | 27/162 (16.7%) | 33/158 (20.9%) | 3/107 (2.8%) |
| Female | 20/67 (29.9%) | 20/64 (31.3%) | 2/44 (4.5%) |
| Race | |||
| White | 35/169 (20.7%) | 37/158 (23.4%) | 5/117 (4.3%) |
| Black or African American | 10/54 (18.5%) | 13/48 (27.1%) | 0 |
| Other | 1/16 (6.3%) | 3/16 (18.8%) | 0 |
| Age Group | |||
| 35 years | 30/128 (23.4%) | 31/139 (22.3%) | 4/93 (4.3%) |
| > 35 years | 17/101 (16.8%) | 22/83 (26.5%) | 1/58 (1.7%) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved LUCEMYRA based primarily on evidence from two clinical trials: Trial 1 (#NCT01863186) and Trial 2 (#NCT00235729) of 866 patients with opioid addiction. The trials were conducted at 31 sites in the United States.
Figures below summarize 866 patients by sex, race, and age who were in the clinical trials used to evaluate the benefit of the drug. This population is called the efficacy population.
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex (efficacy population)

FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate efficacy.
Figure 2. Baseline Demographics by Race (efficacy population)
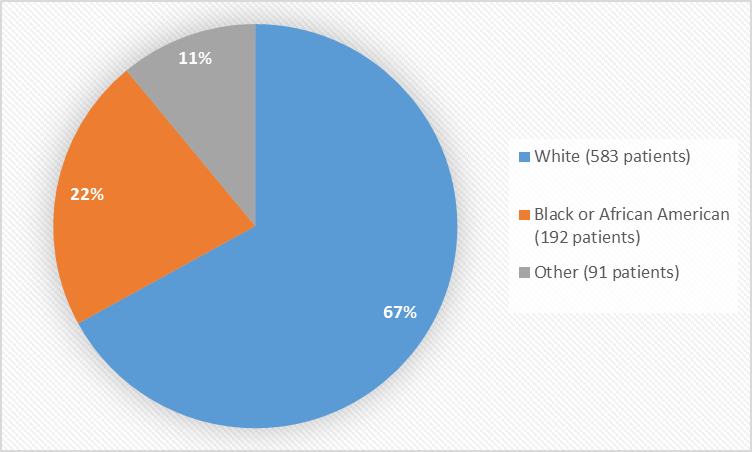
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 583 | 67% |
| Black or African American | 192 | 22% |
| Asian | 5 | Less than 1% |
| American Indian or Alaskan Native | 4 | Less than 1% |
| Native Hawaiin or Other Pacific Islander | 5 | Less than 1% |
| All Other | 77 | 9% |
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age (efficacy population)
Who participated in the trials?
The table below summarizes patients who participated in the combined Trials 1 and 2.
Table 8. Baseline Demographics for Patients in LUCEMYRA Trials 1 and 2 (efficacy population)
| Characteristic | Trial 1 | Trial 2 | TOTAL (N=866) |
|||
|---|---|---|---|---|---|---|
| LUCEMYRA 2.16 mg (N=229) |
LUCEMYRA 2.88 mg (N=222) |
PLACEBO (N=151) |
LUCEMYRA 2.88 mg (N=134) |
PLACEBO (N=130) |
||
| Sex, n (%) | ||||||
| Male | 162 (71%) | 158 (71%) | 107 (71%) | 101 (75%) | 99 (76%) | 627 (72%) |
| Female | 67 (29%) | 64 (29%) | 44 (29%) | 33 (25%) | 31 (24%) | 239 (28%) |
| Race, n (%) | ||||||
| White | 169 (74%) | 158 (71%) | 117 (78%) | 63 (47%) | 76 (59%) | 583 (67%) |
| Black or African American | 54 (24%) | 48 (22%) | 26 (17%) | 37 (28%) | 27 (21%) | 192 (22%) |
| Asian | 1 ( | 3 (1%) | 1 (1%) | 0 | 0 | 5 ( |
| Native Hawaiian or other Pacific Islander | 0 | 2 (1%) | 3 (2%) | 0 | 0 | 5 ( |
| American Indian or Alaska Native | 0 | 2 (1%) | 2 (1%) | 0 | 0 | 4 ( |
| Other | 5 (2%) | 9 (4%) | 2 (1%) | 34 (25%) | 27 (21%) | 77 (9%) |
| Age overall, years | ||||||
| Mean (SD) | 35 (11) | 35 (11) | 36 (12) | 36 (11) | 38 (11) | 36 (11) |
| Median (min, max) | 34 (19, 74) | 33 (19, 68) | 32 (19,63) | 35 (19, 63) | 35 (18, 63) | 35 (18, 74) |
| Age Group, n (%) | ||||||
| 35years | 128 (56%) | 139 (63%) | 93 (62%) | 67 (50%) | 59 (45%) | 486 (56%) |
| > 35 years | 101(44%) | 83 (7%) | 58 (38%) | 67 (50%) | 71 (55%) | 380 (44%) |
| 227 (99%) | 221 (99%) | 151 (100%) | 134 (100%) | 130 (100%) | 863 (100%) | |
| > 65 years | 2 (1%) | 1 ( | 0 | 0 | 0 | 3 ( |
| Ethnicity | ||||||
| Hispanic/Latino | 33 (14%) | 28 (13%) | 22 (15%) | 34 (25%) | 27 (21%) | 144 (17%) |
| Not Hispanic/Latino | 196 (86%) | 194 (87%) | 129 (85%) | 100 (75%) | 103 (89%) | 722 (83%) |
| Geographic Region | ||||||
| USA | 585 (100%) | 356 (100%) | 151 (100%) | 134 (100%) | 281(100%) | 866 (100%) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of LUCEMYRA were primarily evaluated in two clinical trials.
Trial 1: Patients with opioid use disorder and physical dependence on opioids were randomly assigned to receive one of two doses of LUCEMYRA or placebo tablets for 7 days. Patients, who completed 7 days of treatment, could receive LUCEMYRA for an additional 7 days. Neither the patients nor health care providers knew which treatment was given until after the trial was completed. The benefit of LUCEMYRA was assessed based on the difference from placebo in the SOWS – Gossop scores from Days 1 – 7.
Short Opiate Withdrawal Scale of Gossop (SOWS – Gossop) is a scale used to evaluate patients with opioid use disorder undergoing abrupt opioid discontinuation.
Trial 2: Patients with opioid use disorder and physical dependence on opioids were randomly assigned to receive LUCEMYRA or placebo tablets for 5 days, followed by an additional 2 days of treatment with placebo. Neither the patients nor health care providers knew which treatment was given until after the trial was completed. The benefit of LUCEMYRA was based on the patient’s assessment of symptom approval (Short Opiate Withdrawal Scale of Gossop) in the SOWS – Gossop scores from Days 1 – 5.
How were the trials designed?
The efficacy and safety of LUCEMYRA were primarily evaluated in two clinical trials. All trials enrolled patients at least 18 years of age, who had opioid use disorder and were physically dependent on opioids.
Trial 1 was an inpatient, randomized, double-blind, placebo-controlled trial. For Days 1 – 7, patients received LUCEMYRA 2.16 mg, 2.88 mg, or matching placebo. After completing 7 days of treatment, patients were eligible to receive open-label treatment with variable dose LUCEMYRA for an additional 7 days as an inpatient or outpatient. The planned primary endpoint was the least squares means difference from placebo in SOWS – Gossop scores from Days 1 – 7.
The SOWS – Gossop scale is a 10-item scale used to evaluate abrupt opioid withdrawal in adults with opioid use disorder. It rates the severity of each withdrawal symptom on a four-point scale.
Trial 2 was an inpatient, randomized, double-blind, placebo-controlled trial. Patients received LUCEMYRA 2.88 mg or matching placebo on Days 1 - 5. On Days 6 – 7, all patients received placebo. The planned primary endpoints were the SOWS – Gossop on Day 3 of treatment and the time to dropout.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
