Drug Trials Snapshots: RHOPRESSA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the RHOPRESSA Package Insert for complete information.
RHOPRESSA (netarsudil)
‘ro-pre-ssa’
Aerie Pharmaceuticals
Approval date: December 18, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RHOPRESSA is a drug for reducing elevated intraocular pressure, when the pressure inside the eye is too high.
How is this drug used?
One drop of RHOPRESSA is applied in the affected eye once daily in the evening.
What are the benefits of this drug?
RHOPRESSA lowers the intraocular pressure.
What are the benefits of this drug (results of trials used to assess efficacy)?
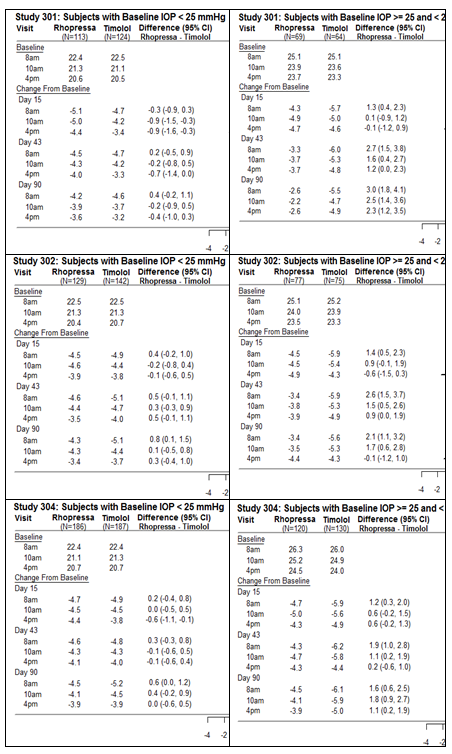
RHOPREESSA lowered intraocular pressure up to 5 mmHg in patients with open-angle glaucoma or ocular hypertension. For patients with baseline IOP < 25="" mmhg,="" the="" iop="" reductions="" with="" rhopressa="" 0.02%="" dosed="" once="" daily="" were="" similar="" to="" those="" with="" timolol="" 0.5%="" dosed="" twice="" daily.="" for="" patients="" with="" baseline="" iop="" equal="" to="" or="" above="" 25="" mmhg,="" however,="" rhopressa="" 0.02%="" resulted="" in="" smaller="" mean="" iop="" reductions="" at="" the="" morning="" time="" points="" than="" timolol="" 0.5%="" for="" study="" visits="" on="" days="" 43="" and="" 90;="" the="" difference="" in="" mean="" iop="" reduction="" between="" the="" two="" treatment="" groups="" was="" as="" high="" as="" 3="" mmhg,="" favoring="" timolol.="" the="" results="" by="" trial="" are="" presented="" in="" the="" figure="">
Figure 4. Mean IOP Change from Baseline of Study Eye (mmHg) by Visit and Time*
*Based on the observed data from all randomized patients who did not have major protocol violations. The treatment differences and two-sided CIs for comparing RHOPRESSA QD vs Timolol BID 0.5% were based on Analysis of Covariance (ANCOVA) adjusted for baseline IOP.
RHOPRESSA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: RHOPRESSA worked similarly in men and women.
- Race: RHOPRESSA worked similarly in White patients and non-White patients (all other races combined).
- Age: RHOPRESSA worked similarly in patients above and below 65 years of age.
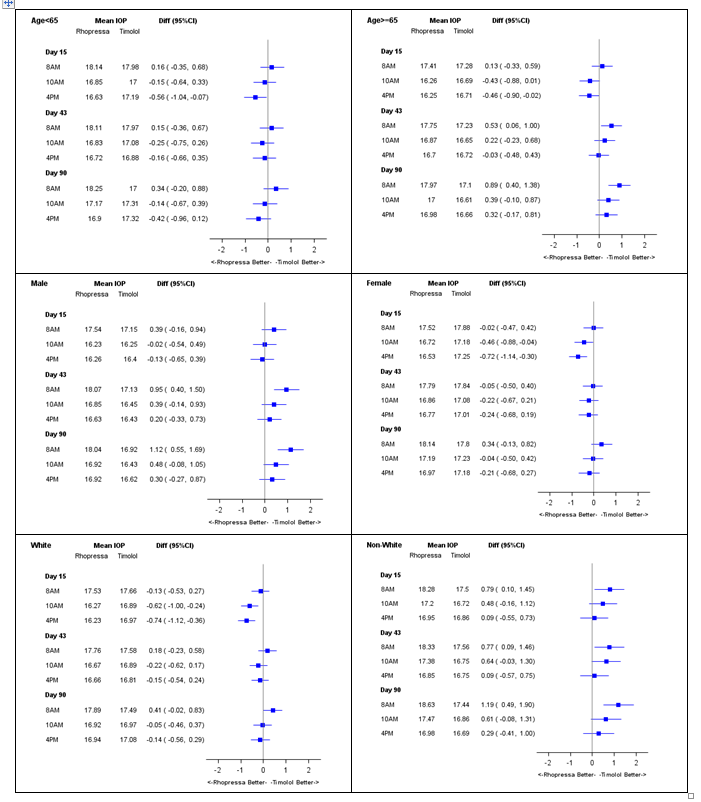
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses based on sex, race, and age are shown in the figures below. The results for each demographic subgroup were generally similar to those seen for the overall population.
Figure 5: Mean IOP Subgroup Analyses by Age, Sex, and Race (Patients with Baseline IOP < 25="" mmhg)="" –="" pooled="" trials="" 301,="" 302="" and="">
FDA Statistics Review
What are the possible side effects?
The most common side effects are conjunctival (eye) redness, golden brown deposits in the cornea (covering of the colored portion of the eye), pain with drug application, conjunctival (eye) bleeding, blurred or decreased vision, increased tearing and redness of the eyelid.
What are the possible side effects (results of trials used to assess safety)?
The most common adverse reaction observed in clinical trials with RHOPRESSA was conjunctival hyperemia which occurred in 53% of patients.
The following adverse reactions occurred in at least 20% of patients:
- Corneal Verticillata
- Instillation site pain
- Conjunctival hemorrhage
The following adverse reactions occurred in approximately 5 to 10%:
- Instillation site erythema
- superficial keratitis
- Blurred vision
- Lacrimation increased
- Erythema of the eyelid
- Decreased visual acuity
RHOPRESSA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of side effects was similar in White and Black or African American patients. The number of patients in other races was limited, therefore differences in side effects among other races could not be determined.
- Age: The risk of side effects was similar in patients above and below 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The frequency of ocular side effects among RHOPRESSA treated patients by sex, race and age are shown in Table 2.
Table 2. Summary of Ocular Adverse Reactions (ARs) in PHOPRESSA Treated Patients by Subgroup
| Ocular ARs | RHOPRESSA N = 1058 x/n (%) |
Timolol N = 816 x/n (%) |
|---|---|---|
| Overall | 791/1058 (75) | 213/816 (26) |
| Sex | ||
| Men | 333/423 (79) | 75/294 (26) |
| Women | 458/635 (72) | 138/522 (26) |
| Age (years) | ||
| <> | 353/480 (74) | 103/390 (26) |
| ≥65 | 438/578 (76) | 110/426 (26) |
| Race | ||
| White | 635/771 (82) | 166/592 (28) |
| Black | 137/265 (52) | 38/202 (19) |
| Asian | 15/17 (88) | 7/16 (44) |
| Other | 4/5 (80) | 2/6 (33) |
x/n= number of patients with at least one ocular adverse reaction (x) in the subgroup (n)
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved RHOPRESSA based on evidence from three clinical trials (NCT # 02207491, NCT# 02207621 and NCT# 02558374) that enrolled 1875 patients with open-angle glaucoma or elevated intraocular pressure. These trials were conducted in the United States.
Figures 1, 2 and 3 summarize patient characteristics in the three clinical trials by sex, race and age.
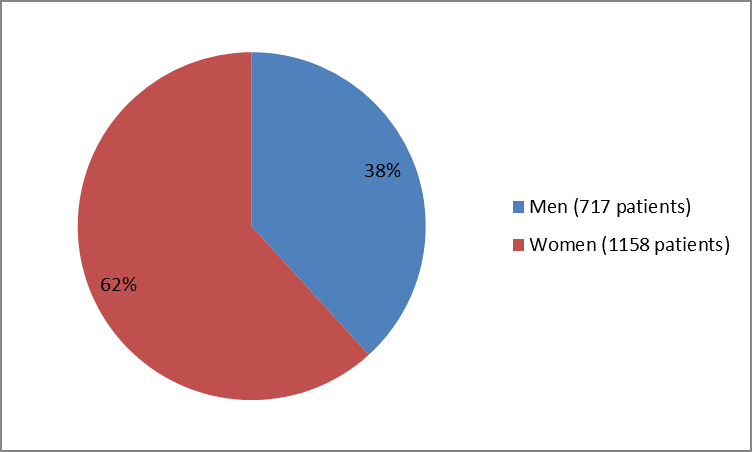
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
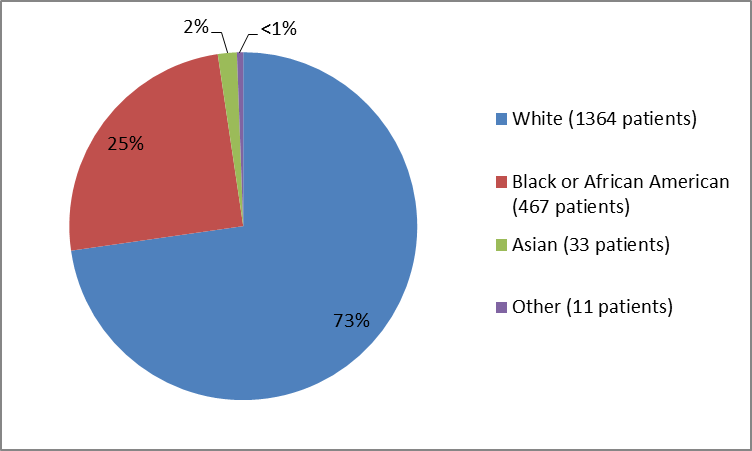
Figure 2. Baseline Demographics by Race
Clinical Trial Data
Table 1. Baseline Demography by Race
| Race | N of Patients | Percentage |
|---|---|---|
| White | 1364 | 73 |
| Black or African American | 467 | 25 |
| Asian | 33 | 2 |
| Other | 11 | less than 1 |
Clinical Trial Data
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics of 1875 patients in the three clinical trials combined.
Table 3: Summary of Demographics – Randomized Population
| Demographic Parameter | N of Patients N = 1875 |
Percentage |
|---|---|---|
| Sex | ||
| Men | 717 | 38 |
| Women | 1158 | 62 |
| Race | ||
| White | 1364 | 73 |
| Black or African American | 467 | 25 |
| Asian | 33 | 2 |
| Other | 11 | <> |
| Age | ||
| Mean (years) | 64.3 | |
| Age Category | ||
| 18-64 years | 871 | 46 |
| 65 years or older | 1004 | 54 |
| Ethnicity | ||
| Hispanic or Latino | 357 | 19 |
| Not Hispanic or Latino | 1518 | 81 |
Clinical Trial Data
How were the trials designed?
Three trials evaluated the benefits and side effects of RHOPRESSA. In each trial, patients were randomly assigned to receive either RHOPRESSA or timolol eye drops (timolol is FDA approved for the treatment of increased intraocular pressure) every day for 3 months. Neither the trial patients nor the health care providers knew which treatment was being given until after the trials were completed. The benefit of RHOPRESSA was measured by decrease in intraocular pressure in comparison to timolol after 2 weeks, 6 weeks and 3 months of treatment.
How were the trials designed?
There were three randomized, multicenter, double-masked, active control trials (NCT # 02207491, NCT# 02207621 and NCT# 02558374) that evaluated the safety and efficacy of RHOPRESSA given once daily. In two trials, patients were randomized in 1:1 ratio to receive either RHOPRESSA once daily or timolol 0.5% twice daily. In the third trial, patients were randomized in 1:1:1 ratio to receive RHOPRESSA once daily, RHOPRESSA twice daily or timolol 0.5% twice daily. The primary efficacy endpoint was change in the mean IOP in the study eye measured at Week 2, Week 6 and Month 3.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION