Drug Trials Snapshots: ROZLYTREK
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to ROZLYTREK Prescribing Information for complete information.
ROZLYTREK (entrectinib)
roz lye' trek
Genentech, Inc.
Approval date: August 15, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ROZLYTREK is a drug used to treat adult patients with a type of non-small cell lung cancer (NSCLC) which:
- is caused by an abnormal ROS1 gene and,
- has spread to other parts of the body (metastatic).
How is this drug used?
ROZLYTREK is a capsule taken by mouth once a day.
What are the benefits of this drug?
Forty (78%) of 51 patients with NSCLC who received ROZLYTREK experienced complete or partial shrinkage of their tumors. Tumor shrinkage lasted more than 12 months for 55% % of those patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results based on overall response rate. Efficacy was assessed in 51 adult patients from three trials with metastatic NSCLC whose tumors were ROS1 positive.
Table 2. Efficacy Results in ROS1-Positive NSCLC Patients per BICR Assessment
| Efficacy Parameters | ROZLYTREK N= 51 |
|---|---|
| Overall Response Rate (95% CI) | 78% (65, 89) |
| Complete Response | 6% |
| Partial Response | 73% |
| Duration of Response (DOR)* | N=40 |
| Range (months) | 1.8,36.8+ |
| % DOR ≥9 months | 70% |
| % DOR ≥12 months | 55% |
| % DOR ≥18 months | 30% |
BIRC= blinded independent review committee; Confidence Interval (CI) calculated using the Clopper-Pearson method.
Response duration were based on additional 5 months’ follow-up after the primary analysis of ORR.* Observed DOR
+ denotes ongoing response
ROZLYTREK Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The difference in how well the drug worked in clinical trials among sex, race and age groups could not be determined because of the small sample sizes.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes overall response rate by sex, race and age subgroups. Results should be interpreted with caution given the small sample size overall, and the limited number of patients in each subgroup.
Table 3. Subgroup Analyses Based on Overall Response Rate
| Subgroup | Patients, n | Responders, n (ORR, %) | 95% CI |
|---|---|---|---|
| Sex | |||
| Men | 17 | 14 (82.4%) | (56.6%, 96.2%) |
| Women | 34 | 26 (76%) | (58.8%, 89.3%) |
| Race | |||
| Asian | 19 | 16 (84%) | (60.4%, 96.6%) |
| White | 29 | 22 (75.9%) | (56.5%, 89.7%) |
| Age | |||
| >=65 | 10 | 8 (80.0%) | (44.4%, 97.5%) |
| <65 | 41 | 32 (78.0%) | (62.4%, 89.4%) |
| Region | |||
| USA | 14 | 11 (78.6%) | (49.2%, 95.3%) |
| Non‐USA | 37 | 29 (78.4%) | (61.8%, 90.2%) |
CI = confidence interval
FDA Review
What are the possible side effects?
ROZLYTREK may cause serious side effects including congestive heart failure, nervous system problems, bone fractures, liver toxicity, increased uric acid in the blood, heart rhythm problems (because of changes in heart electrical activity called QT prolongation) and vision problems.
The most common side effects of ROZLYTREK are tiredness, constipation, taste changes, body swelling, dizziness, and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in ≥10% of patients treated with ROZLYTREK combining data from four clinical trials.
Table 4. Adverse Reactions Occurring in ≥10% of Patients Treated with ROZLYTREK
| Adverse Reactions | ROZLYTREK N=355 |
|
|---|---|---|
| All Grades (%) | Grade ≥ 3* (%) | |
| General | ||
| Fatigue1 | 48 | 5 |
| Edema2 | 40 | 1.1 |
| Pyrexia | 21 | 0.8 |
| Gastrointestinal | ||
| Constipation | 46 | 0.6 |
| Diarrhea | 35 | 2.0 |
| Nausea | 34 | 0.3 |
| Vomiting | 24 | 0.8 |
| Abdominal pain3 | 16 | 0.6 |
| Nervous System | ||
| Dysgeusia | 44 | 0.3 |
| Dizziness4 | 38 | 0.8 |
| Dysesthesia5 | 34 | 0.3 |
| Cognitive effects6 | 27 | 4.5 |
| Peripheral sensory neuropathy7 | 18 | 1.1 |
| Headache | 18 | 0.3 |
| Ataxia8 | 17 | 0.8 |
| Sleep9 | 14 | 0.6 |
| Mood disorders10 | 10 | 0.6 |
| Respiratory, Thoracic and Mediastinal | ||
| Dyspnea | 30 | 6* |
| Cough | 24 | 0.3 |
| Metabolism and Nutritional | ||
| Increased Weight | 25 | 7 |
| Decreased appetite | 13 | 0.3 |
| Dehydration | 10 | 1.1 |
| Musculoskeletal and Connective Tissue | ||
| Myalgia11 | 29 | 1.1 |
| Arthralgia | 21 | 0.6 |
| Muscular weakness | 12 | 0.8 |
| Back pain | 12 | 1 |
| Pain in extremity | 11 | 0.3 |
| Metabolism and Nutritional | ||
| Increased weight | 25 | 7 |
| Decreased appetite | 13 | 0.3 |
| Dehydration | 10 | 1.1 |
| Eye | ||
| Vision disorders12 | 21 | 0.8 |
| Infections | ||
| Urinary tract infection | 13 | 2.3 |
| Lung infection13 | 10 | 6* |
| Vascular | ||
| Hypotension14 | 18 | 2.8 |
| Skin and Subcutaneous Tissue | ||
| Rash15 | 11 | 0.8 |
* Grades 3 – 5, inclusive of fatal adverse reactions, including 2 events of pneumonia and 2 events of dyspnea.
1Includes fatigue, asthenia
2Includes face edema, fluid retention, generalized edema, localized edema, edema, edema peripheral, peripheral swelling
3Includes abdominal pain upper, abdominal pain, lower abdominal discomfort, abdominal tenderness
4Includes dizziness, vertigo, dizziness postural 5Includes paresthesia, hyperesthesia, hypoesthesia, dysesthesia, oral hypoesthesia, palmar-plantar erythrodysesthesia, oral paresthesia, genital hypoesthesia
6Includes amnesia, aphasia, cognitive disorder, confusional state, delirium, disturbance in attention, hallucinations, visual hallucination, memory impairment, mental disorder, mental status changes
7Includes neuralgia, neuropathy peripheral, peripheral motor neuropathy, peripheral sensory neuropathy
8Includes ataxia, balance disorder, gait disturbances
9Includes hypersomnia, insomnia, sleep disorder, somnolence
10Includes anxiety, affect lability, affective disorder, agitation, depressed mood, euphoric mood, mood altered, mood swings, irritability, depression, persistent depressive disorder, psychomotor retardation
11Includes musculoskeletal pain, musculoskeletal chest pain, myalgia, neck pain
12 Includes blindness, cataract, cortical cataract, corneal erosion, diplopia, eye disorder, photophobia, photopsia, retinal hemorrhage, vision blurred, visual impairment, vitreous adhesions, vitreous detachment, vitreous floaters
13 Includes lower respiratory tract infection, lung infection, pneumonia, respiratory tract infection
14 Includes hypotension, orthostatic hypotension
15 Includes rash, rash maculopapular, rash pruritic, rash erythematous, rash papular
The table below summarizes laboratory abnormalities that occurred in ≥ 20% patients treated with ROZLYTREK.
Table 5. Laboratory Abnormalities (≥ 20%) Worsening from Baseline in Patients Receiving ROZLYTREK (safety population)
| Laboratory Abnormality | ROZLYTREK NCI CTCAE Grade |
|
|---|---|---|
| All Grades (%)1 | Grade 3 or 4 (%)1 | |
| Hematology | ||
| Anemia | 67 | 9 |
| Lymphopenia | 40 | 12 |
| Neutropenia | 28 | 7 |
| Chemistry | ||
| Increased creatinine2 | 73 | 2.1 |
| Hyperuricemia | 52 | 10 |
| Increased AST | 44 | 2.7 |
| Increased ALT | 38 | 2.9 |
| Hypernatremia | 35 | 0.9 |
| Hypocalcemia | 34 | 1.8 |
| Hypophosphatemia | 30 | 7 |
| Increased lipase | 28 | 10 |
| Hypoalbuminemia | 28 | 2.9 |
| Increased amylase | 26 | 5.4 |
| Hyperkalemia | 25 | 1.5 |
| Increased alkaline phosphatase | 25 | 0.9 |
| Hyperglycemia3 | NE3 | 3.8 |
AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase
1 Denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available which ranged from 111 to 346 patients.
2 Based on NCI CTCAE v5.0
3 NE = Not evaluable. Grade 1 and 2 could not be determined per NCI CTCAE v5.0, as fasting glucose values were not collected
ROZLYTREK Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority patients in the clinical trial were White or Asian. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 summarizes selected adverse events that occurred in the clinical trials by subgroups.
Table 6. Subgroup Analysis of Adverse Events (safety population)
| All (N=355) % |
Sex | Race | Age | ||||
|---|---|---|---|---|---|---|---|
| Men n=161 % |
Women n=194 % |
White n=235 % |
Asian n=82 % |
<65 years n=265 % |
≥65 years n=90 % |
||
| Any Adverse Event | 99 | 99 | 100 | 99 | 100 | 100 | 99 |
| Serious Adverse Event | 39 | 37 | 40 | 48 | 39 | 40 | 33 |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ROZLYTREK based on the evidence from four clinical trials of patients with various types of solid tumors: Trial 1 (EudraCT 2012-000148-88), Trial 2 (NCT02097810), Trial 3 (NCT02568267), and Trial 4 (NCT02650401). The trials were conducted in the United States, Europe and Asia/Pacific region.
A subgroup of 51 patients with NSCLC from Trials 1, 2 and 3 that provided data characterizing the benefit of ROZLYTREK (efficacy population) is presented in Table 7 under MORE INFO.
All 355 patients (regardless of tumor types) from the four trials that provided data for the side effects of ROZLYTREK (safety population) are presented below.
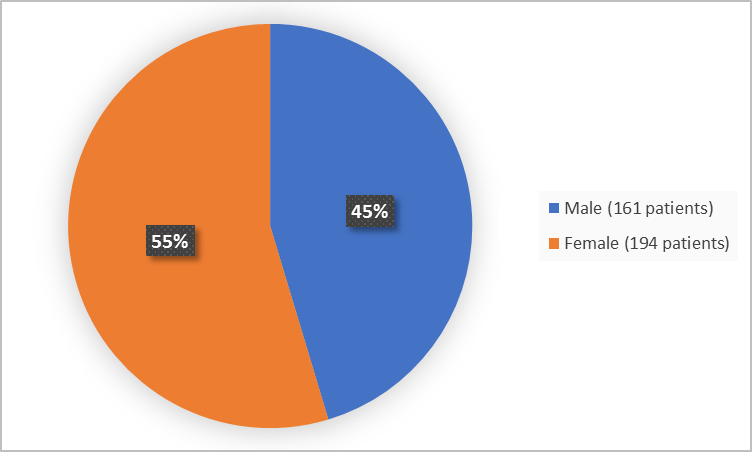
Figure 1 below summarizes how many patients were in the clinical trials by sex.
Figure 1. Baseline Demographics by Sex (safety population)
Clinical Trial Data
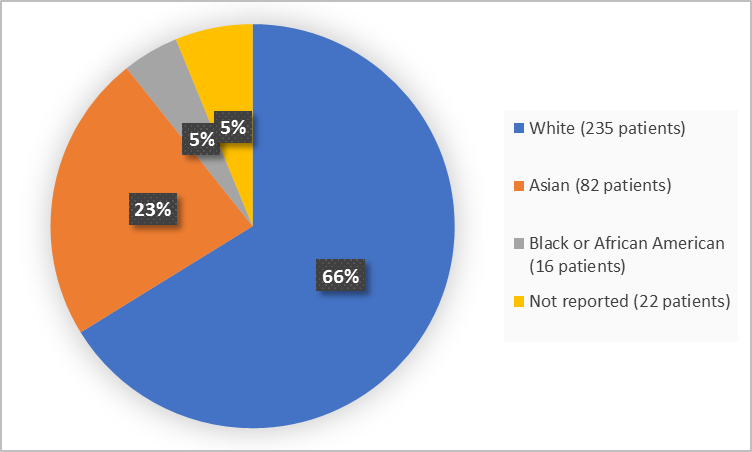
Figure 2 and Table 1 below summarize the percentage of patients in the clinical trials by race.
Figure 2. Baseline Demographics by Race (safety population)
Clinical Trial Data
Table 1. Demographics of Trial by Race (safety population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 235 | 66 |
| Black or African American | 16 | 5 |
| Asian | 82 | 23 |
| Not Reported | 22 | 5 |
Clinical Trial Data
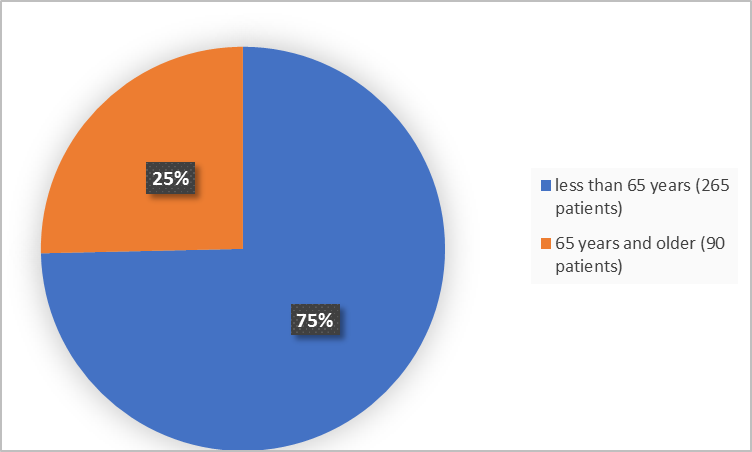
Figure 3 below summarizes the percentage of patients in the clinical trials by age.
Figure 3. Baseline Demographics by Age (safety population)
Clinical Trial Data
Who participated in the trials?
Table 7 below summarizes patient demographics for the efficacy population of patients with ROS1-positive NSCLC and for the safety population of 355 patients who received at least one dose of ROZLYTREK irrespective of tumor type.
Table 7. Baseline Demographics of Patients in the Clinical Trials
| Demographic Parameter | Efficacy Population N=51 n (%) |
Safety Population N=355 n (%) |
|---|---|---|
| Sex, n (%) | ||
| Men | 17 (33) | 161 (45) |
| Women | 34 (67) | 194 (55) |
| Race, n (%) | ||
| White | 29 (57) | 235 (66) |
| Black or African American | 3 (6) | 16 (4.5) |
| Asian | 19 (37) | 82 (23) |
| Not Reported/Other | 0 | 21 (4.5) |
| Age (years) | ||
| Range | 27,72 | 4,86 |
| Median | 53 | 55 |
| Age Group, n (%) | ||
| <65 years | 10 (20) | 265 (75) |
| ≥65 years | 41 (80) | 90 (25) |
| Ethnicity, n (%) | ||
| Hispanic | 2 (4) | 10 (3) |
| Non-Hispanic | 38 (75) | 264 (89) |
| Unknown/Not Reported | 11 (22) | 23 (8) |
| Geographic Region, n (%) | ||
| North America | 14 (28) | 180 (51) |
| Europe | 19 (36) | 102 (29) |
| Asia/Pacific | 18 (36) | 73 (20) |
Clinical Trial Data
How were the trials designed?
The side effects of ROZLYTREK for NSCLC were evaluated in all patients enrolled in four clinical trials, and the benefit was evaluated in a subgroup of patients enrolled in three clinical trials.
Most adults received 600mg ROZLYTREK orally once a day until either tumor progression or intolerable side effects.
The benefit of ROZLYTREK for the treatment of NSCLC was evaluated by measuring the percentage of patients who achieved complete or partial shrinkage of their tumors (overall response rate) and by measuring the duration of that benefit (duration of response).
How were the trials designed?
There were three single-arm, open-label trials that provided data to evaluate the efficacy ROZLYTREK for patients with recurrent or metastatic ROS-1 positive NSCLC. The majority of the 51 patients received ROZLYTREK 600 mg orally once daily until unacceptable toxicity or disease progression.
The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR), as determined by a blinded independent review committee (BIRC) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
Safety assessment was done on all patients from these three trials who received at least one dose of ROZLYTREK and one additional trial that enrolled pediatric and adult patients 4 to 20 years of age.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.