Drug Trials Snapshots: RUKOBIA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the RUKOBIA Prescribing Information for complete information.
RUKOBIA (fostemsavir)
rue-KOH-bee-ah
ViiV Healthcare
Approval date: July 2, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RUKOBIA is a drug for the treatment of human immunodeficiency virus-1 (HIV-1) infection in adults who
- have received several anti-HIV-1 regimens in the past, and
- have HIV-1 virus that is resistant to many antiretroviral medicines, and
- who are failing their current antiretroviral therapy because it is not effective, have intolerable side effects or other safety problems.
HIV-1 is the virus that causes acquired immune deficiency syndrome (AIDS).
How is this drug used?
RUKOBIA is a tablet that is taken by mouth twice a day in combination with other drugs approved for the treatment of HIV-1 infection.
What are the benefits of this drug?
RUKOBIA reduced viral load of HIV-1.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the primary efficacy endpoint defined as the adjusted mean decline in HIV-1 RNA from Day 1 to Day 8 with RUKOBIA versus placebo in the randomized cohort.
Table 1. Plasma HIV-1 RNA Log10 (copies/mL) Change from Day 1 at to Day 8 (Randomized Cohort)– ITT-E Population
|
|
RUKOBIA |
Placebo |
|
Adjusted Meanb (95% CI) |
-0.791 |
-0.166 |
|
Differencec (95% CI) |
-0.625 |
|
a Two patients who received RUKOBIA with missing Day 1 HIV-1 RNA values were not included in the analysis.
b Mean adjusted by Day 1 log10 HIV-1 RNA.
c Difference: RUKOBIA minus placebo.
d P-value <0.0001 for the adjusted and unadjusted mean difference of viral load change from baseline for RUKOBIA compared to placebo.
RUKOBIA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: RUKOBIA worked similarly in men and women.
- Race: RUKOBIA worked similarly in all races.
- Age: The majority of patients in the trials were below 65 years of age. Differences in how well RUKOBIA worked between those below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses of the primary endpoint are presented below.
Table 2. Primary Analysis by Sex Group
|
Sex |
RUKOBIA |
Placebo |
Difference (95%CI) |
||
|
n |
Adjusted Mean |
n |
Adjusted Mean |
||
|
Men |
141 |
-0.82 (-0.92, -0.71) |
57 |
-0.14 (-0.31, +0.03) |
-0.68 (-0.88, -0.48) |
|
Women |
60 |
-0.74 (-0.93, -0.55) |
12 |
-0.29 (-0.71, +0.14) |
-0.45 (-0.92, +0.01) |
Table 3. Primary Analysis by Race Group
|
Race |
RUKOBIA |
Placebo |
Difference (95%CI) |
||
|
n |
Adjusted Mean |
n |
Adjusted Mean |
||
|
White |
135 |
-0.76 (-0.88, -0.64) |
48 |
-0.20 (-0.41, -0.00) |
-0.56 (-0.80, -0.33) |
|
Black or African American |
42 |
-0.82 (-1.00, -0.63) |
18 |
-0.05 (-0.34, +0.23) |
-0.76 (-1.1, -0.42) |
|
Other |
24 |
-0.92 (-1.18, -0.66) |
3 |
-0.11 (-0.84, +0.62) |
-0.81 (-1.58, -0.03) |
Table 4. Primary Analysis by Age Group
|
Age Group |
RUKOBIA |
Placebo |
Difference (95%CI) |
||
|
n |
Adjusted Mean |
n |
Adjusted Mean |
||
|
<35 years |
45 |
-0.75 (-0.94, -0.57) |
16 |
-0.24 (-0.56, +0.07) |
-0.51 (-0.87, -0.15) |
|
35-49 years |
70 |
-0.82 (-1.00, -0.64) |
30 |
-0.05 (-0.33, +0.23) |
-0.77 (-1.10, -0.44) |
|
≥50 years |
86 |
-0.80 (-0.93, -0.67) |
23 |
-0.22 (-0.48, +0.03) |
-0.58 (-0.87, -0.29) |
Adapted from FDA Review
What are the possible side effects?
RUKOBIA may cause a serious condition called immune reconstitution syndrome, similar to other approved drugs for treatment of HIV-1 infection. This condition can happen at the beginning of HIV-1 treatment when the immune system may get stronger and begin to fight infections that have been hidden in the body for a long time. Other serious side effects include heart rhythm problems due to prolongation of heart electrical activity (QT prolongation) and an increase of liver enzymes in patients with hepatitis B or C virus co-infection.
The most common side effect associated with RUKOBIA was nausea.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions reported in greater than or equal to 2% of patients in randomized cohort during 96 weeks of exposure.
Table 5. Adverse Reactionsa (Grades 1 to 4) Reported in ≥2% of Subjects Receiving RUKOBIA plus OBT in the Trial, Randomized Cohort (Week 96 Analysis)
|
Adverse Reaction |
RUKOBIA plus OBT |
|
Nausea |
10% |
|
Diarrhea |
4% |
|
Headache |
4% |
|
Abdominal pain |
3% |
|
Dyspepsia |
3% |
|
Fatigue |
3% |
|
Rash |
3% |
|
Sleep disturbancef |
3% |
|
Immune Reconstitution Inflammatory Syndrome |
2% |
|
Somnolence |
2% |
|
Vomiting |
2% |
a Frequencies of adverse reactions are based on all treatment-emergent adverse events attributed to study drug by the investigator.
b Of the 272 subjects enrolled in the randomized cohort, 1 subject who received placebo withdrew from the trial prior to receiving RUKOBIA in the open-label phase of the trial.
c Includes pooled terms: fatigue and asthenia.
d Includes pooled terms: rash, rash generalized, rash maculo-papular, rash pruritic, and dermatitis allergic.
e Includes pooled terms: abdominal discomfort, abdominal pain, and abdominal pain upper.
f Includes pooled terms: insomnia, sleep deficit, sleep disorder, abnormal dreams.
| Adverse reactions in the nonrandomized cohort were similar to those observed in the randomized cohort. The most common adverse reactions reported in nonrandomized subjects were fatigue (7%), nausea (6%), and diarrhea (6%). |
RUKOBIA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all races.
- Age: The majority of patients in the trials were below 65 years of age. Differences in the occurrence of side effects between those below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of adverse events in randomized population during 96 weeks of RUKOBIA plus OBT therapy.
Table 6. Adverse Events (AEs) by Demographic Subgroups
|
Adverse Event |
Total N=272 |
Sex |
Race |
Age (years) |
|||||
|
Men |
Women |
White |
Black |
All Other |
<35 |
35 – 49 |
≥50 |
||
|
Any AE |
249 (92) |
182 (91) |
67 (93) |
170 (92) |
55 (92) |
24 (89) |
55 (90) |
90 (89) |
104 (95) |
|
Grade 3-4 AE |
79 (29) |
62 (31) |
17 (24) |
59 (32) |
14 (23) |
6 (22) |
15 (25) |
24 (24) |
40 (36) |
|
SAE |
92 (34) |
73 (37) |
19 (26) |
63 (34) |
21 (35) |
8 (30) |
18 (30) |
28 (28) |
46 (42) |
Adapted from FDA Review
WHO WAS IN THE CLINCAL TRIALS?
Who participated in the clinical trials?
The FDA approved RUKOBIA based on evidence from one clinical trial (NCT02362503) which enrolled adults with HIV-1 infection who have received prior treatments for the infection. Trial was conducted at 108 sites in 23 countries in North America, South America, Europe, Australia, Taiwan and South Africa.
Demographics of all 272 randomized patients from the trial are presented in the Figures 1-4 below. Demographics of 99 non-randomized patients who also participated in the trial are presented separately in Table 7, under MORE INFO.
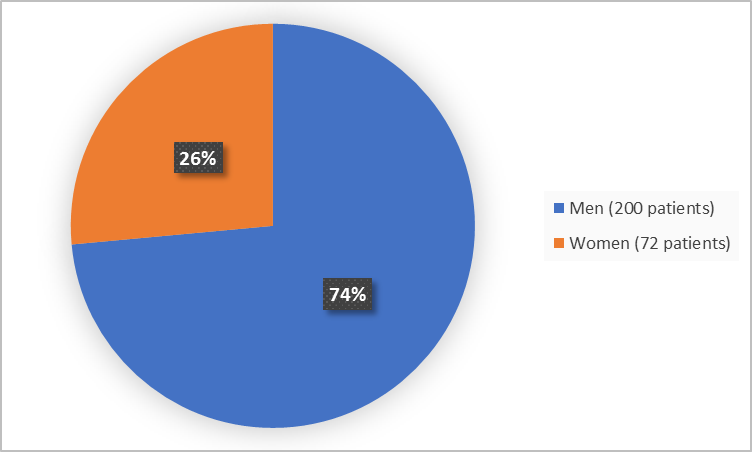
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex (randomized population)
FDA Review
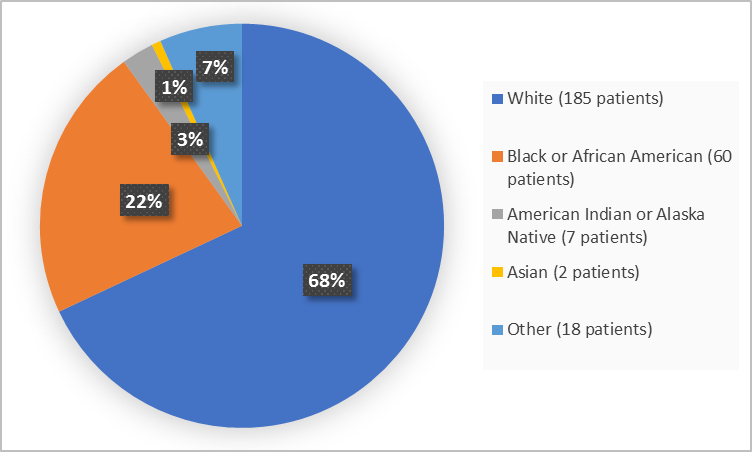
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race (randomized population)
FDA Review
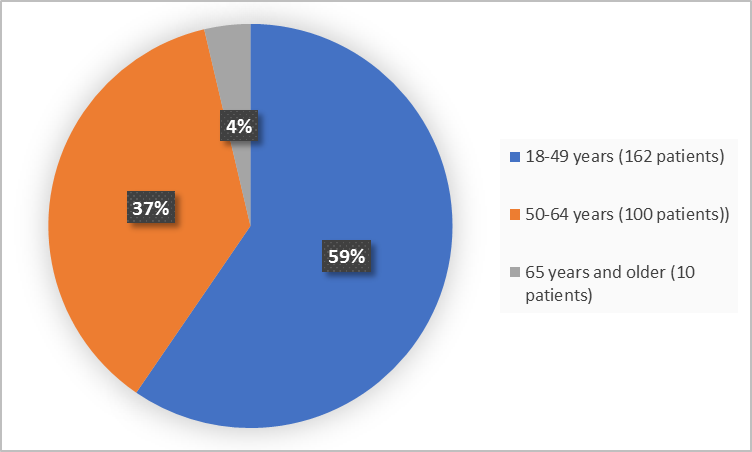
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Demographics by Age (randomized population)
FDA Review
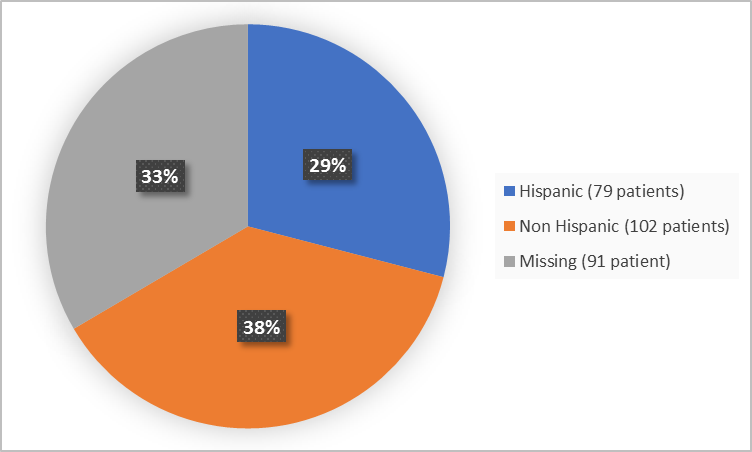
Figure 4 summarizes patients by ethnicity in the clinical trial.
Figure 4. Demographics by Ethnicity(randomized population)
FDA Review
Who participated in the trials?
The table below summarizes trial demographics.
Table 7. Trial Demographics
|
Demographic Characteristic |
Randomized Cohort |
Non-Randomized Cohort |
||
|
RUKOBIA |
Placebo |
Total |
||
|
Sex, n (%) |
|
|
|
|
|
Men |
143 (70) |
57 (83) |
200 (74) |
89 (90) |
|
Women |
60 (30) |
12 (17) |
72 (26) |
10 (10) |
|
Race, n (%) |
|
|
|
|
|
White |
137 (67) |
48 (70) |
185 (68) |
74 (75) |
|
Black or African American |
42 (21) |
18 (26) |
60 (22) |
23 (23) |
|
American Indian or Alaska Native |
6 (3) |
1 (1) |
7 (3) |
1 (1) |
|
Asian |
2 (1) |
0 |
2 (1) |
0 |
|
Other |
16 (8) |
2 (3) |
18 (7) |
1 (1) |
|
Age, years |
|
|
|
|
|
Mean (SE) |
45 (1) |
43 (1) |
45 (1) |
48 (1) |
|
Median (min, max) |
48 (18, 73) |
45 (19, 66) |
48 (18, 73) |
50 (17, 72) |
|
Age groups, n (%) |
|
|
|
|
|
<35 years |
45 (22) |
16 (23) |
61 (22) |
14 (14) |
|
35-49 years |
71 (35) |
30 (43) |
101 (37) |
30 (30) |
|
≥50 years |
87 (43) |
23 (33) |
110 (40) |
55 (56) |
|
Ethnicity, n (%) |
|
|
|
|
|
Hispanic |
61 (30) |
18 (26) |
79 (29) |
28 (28) |
|
Non-Hispanic |
76 (37) |
26 (38) |
102 (38) |
53 (54) |
|
Missing |
66 (33) |
25 (36) |
91 (33) |
18 (18) |
|
Region, n (%) |
|
|
|
|
|
North America |
79 (39) |
29 (42) |
108 (40) |
56 (57) |
|
USA |
62 (31) |
24(35) |
86 (32) |
50 (51) |
|
South America |
80 (39) |
25 (36) |
105 (39) |
14 (14) |
|
Europe |
38 (19) |
13 (19) |
51 (19) |
27 (27) |
|
Rest of World |
6 (3) |
2 (3) |
8 (3) |
2 (2) |
Adapted from FDA Review
How were the trials designed?
RUKOBIA was evaluated in one clinical trial of adults with HIV-1 infection who have taken medicines to treat the infection before but those did not work well anymore.
A larger group of patients (randomized cohort) received either RUKOBIA or placebo twice daily in addition to their current failing regimen. The trial measured viral decline in the plasma from the first day of treatment to day 8 and compared RUKOBIA to placebo. After the first 8 days, all patients received RUKOBIA plus an optimized background therapy (OBT) selected by the investigator and primarily provided data for the evaluation of side effects.
There was also a separate smaller group of patients with advanced HIV-1 infection and who did not have any remaining fully active approved antiretroviral therapy options (non-randomized cohort). These patients received RUKOBIA daily plus OBT from Day 1 onward. Data from this group provided supportive evidence for RUKOBIA side effects.
How were the trials designed?
FDA approved RUKOBIA based on data from one partially randomized, double-blind, placebo-controlled, international, multicenter trial conducted in heavily treatment-experienced adult patients.
In the randomized cohort, patients received either RUKOBIA or placebo twice daily in addition to their current failing HIV-1 regimen. The primary efficacy endpoint was the adjusted mean decline in HIV-1 RNA from Day 1 to Day 8. Beyond Day 8 though Week 96, all patients received open-label RUKOBIA plus an investigator-selected OBT.
In the non-randomized cohort, patients had no fully active and approved antiretroviral agent(s) available at screening and received open-label RUKOBIA twice daily plus OBT from Day 1 onward.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.