Drug Trials Snapshots: SARCLISA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the SARCLISA Prescribing Information for complete information.
SARCLISA (isatuximab-irfc)
(sar-cli-sa)
Sanofi-Aventis U.S. LLC
Approval date: March 2, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SARCLISA is a drug used to treat a form of blood cancer called multiple myeloma. SARCLISA is for adult patients whose cancer returned or did not respond to, at least two previous treatments for multiple myeloma.
It is to be used in combination with two other drugs:
- dexamethasone (a type of corticosteroid) and
- pomalidomide.
How is this drug used?
SARCLISA is an injection. It is given by a healthcare profesional directly into the vein (intravenous infusion) every week for 4 weeks followed by every 2 weeks.
What are the benefits of this drug?
In the trial, patients who received SARCLISA (in combination with pomalidomadie and dexamethasone) lived longer without the cancer growing (average about 12 months) in comparison to patients who received pomalidomadie and dexamethasone combination only (average about 6 months).
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of SARCLISA was based upon progression-free survival (PFS).
Table 1. Efficacy of SARCLISA in Combination with Pomalidomide and Low-Dose Dexamethsone in the Treatment of Multiple Myeloma
|
Endpoint |
SARCLISA + Pomalidomide + Dexamethasone |
Pomalidomide + Dexamethasone |
|
Progression-Free Survival |
||
|
Median (months) [95% CI] |
11.53 |
6.47 |
|
Hazard ratioa [95% CI] |
0.596 [0.44-0.81] |
|
|
p-valuea (stratified log-rank test) |
0.0010 |
|
|
Overall Response Rateb |
93 (60.4) |
54 (35.3) |
|
p-value (stratified Cochran-Mantel-Haenszel)a |
<0.0001 |
|
|
Stringent Complete Response (sCR) + |
7 (4.5) |
3 (2) |
|
Very Good Partial Response (VGPR) n (%) |
42 (27.3) |
10 (6.5) |
|
Partial Response (PR) n (%) |
44 (28.6) |
41 (26.8) |
a Stratified on age (<75 years versus ≥75 years) and number of previous lines of therapy (2 or 3 vs >3) according to IRT.
b sCR, CR, VGPR and PR were evaluated by the IRC using the IMWG response criteria.
c Estimated using Clopper-Pearson method.
SARCLISA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: SARCLISA worked similarly in men and women.
- Race: The majority of patients were White. Differences in response to SARCLISA among different races could not be determined.
- Age: SARCLISA worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes subgroup analyses of PFF in the efficacy population of Trial 1.
Table 2. Subgroup Analyses of Progression Free Survival
|
|
SARCLISA + Pomalidomide + Dexamethasone |
Pomalidomide + Dexamethasone |
HR (95% CI) |
||||
|
N |
N (%) of Events |
Median (months) |
N |
N (%) of Events |
Median (months) |
||
|
All patients |
154 |
73 (47.4) |
11.53 |
153 |
89 (58.2) |
6.47 |
0.6 (0.44 to 0.81) |
|
Sex |
|
|
|
|
|
|
|
|
Men |
89 |
43 (48.3) |
11.50 |
70 |
41 (58.6) |
6.47 (3.75,11.07) |
0.67 (0.44, 1.02) |
|
Women |
65 |
30 (46.2) |
12.72 (7.03,15.21) |
83 |
48 (57.8) |
7.39 |
0.55 (0.35, 0.877) |
|
Race |
|
|
|
|
|
|
|
|
White |
118 |
56 (47.5) |
11.53 (8.97,14.78) |
126 |
74 (58.7) |
6.47 |
0.59 (0.41, 0.83) |
|
Asian |
21 |
8 (38.1) |
NC (5.81,NC) |
15 |
8 (53.3) |
7.85 |
0.52 (0.19, 1.39) |
|
Age (years) |
|
|
|
|
|
|
|
|
<65 |
54 |
26 (48.1) |
11.53 (4.57,14.78) |
70 |
41 (58.6) |
5.03 |
0.66 (0.4 ,1.07) |
|
65-75 |
68 |
32 (47.1) |
11.57 |
54 |
29 (53.7) |
8.58 (4.57,12.06) |
0.64 (0.39, 1.06) |
|
≥75 |
32 |
15 (46.9) |
11.40 |
29 |
19 (65.5) |
4.47 |
0.48 (0.24, 0.95) |
NC=Not calculable
Adapted from FDA Review
What are the possible side effects?
SARCLISA may cause serious side effects including infusion reactions, low neutrophil count (neutropenia), and possible development of new cancers. The most common side effects of SARCLISA are low blood counts, infusion reactions, pneumonia, upper respiratory infections and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize the adverse reactions and laboratory abnormalities that occurred during the trial.
Table 3. Adverse Reactions (≥10%) in Patients Receiving SARCLISA, Pomalidomide, and Dexamethasone with a Difference Between Arms of ≥5% Compared to Control Arm
|
Adverse Reactions |
SARCLISA + Pomalidomide + Dexamethasone |
Pomalidomide + Dexamethasone (N=149) |
||||
|
All grades (%) |
Grade 3 (%) |
Grade 4 (%) |
All grades (%) |
Grade 3 (%) |
Grade 4 (%) |
|
|
Infusion-related reaction |
38 |
1.3 |
1.3 |
0 |
0 |
0 |
|
Infections |
||||||
|
Pneumoniaa |
31 |
22 |
3.3 |
23 |
16 |
2.7 |
|
Upper respiratory tract infectionb |
57 |
9 |
0 |
42 |
3.4 |
0 |
|
Blood and lymphatic system disorders |
||||||
|
Febrile neutropenia |
12 |
11 |
1.3 |
2 |
1.3 |
0.7 |
|
Respiratory, thoracic and mediastinal disorders |
||||||
|
Dyspneac |
17 |
5.0 |
0 |
12 |
1.3 |
0 |
|
Gastrointestinal disorders |
||||||
|
Diarrhea |
26 |
2 |
– |
19 |
0.7 |
– |
|
Nausea |
15 |
0 |
– |
9 |
0 |
– |
|
Vomiting |
12 |
1.3 |
– |
3.4 |
0 |
– |
a Pneumonia includes atypical pneumonia, bronchopulmonary aspergillosis, pneumonia, pneumonia haemophilus, pneumonia influenzal, pneumonia pneumococcal, pneumonia streptococcal, pneumonia viral, candida pneumonia, pneumonia bacterial, haemophilus infection, lung infection, pneumonia fungal, and pneumocystis jirovecii pneumonia.
b Upper respiratory tract infection includes bronchiolitis, bronchitis, bronchitis viral, chronic sinusitis, fungal pharyngitis, influenza-like illness, laryngitis, nasopharyngitis, parainfluenzae virus infection, pharyngitis, respiratory tract infection, respiratory tract infection viral, rhinitis, sinusitis, tracheitis, upper respiratory tract infection, and upper respiratory tract infection bacterial.
c Dyspnea includes dyspnea, dyspnea exertional, and dyspnea at rest.
Table 4: Treatment Emergent Hematology Laboratory Abnormalities
|
Laboratory Parameter n (%) |
SARCLISA + Pomalidomide + Dexamethasone |
Pomalidomide + Dexamethasone (N=149) |
||||
|
All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|
|
Anemia |
151 (99) |
48 (32) |
0 |
145 (97) |
41 (28) |
0 |
|
Neutropenia |
146 (96) |
37 (24) |
92 (61) |
137 (92) |
57 (38) |
46 (31) |
|
Lymphopenia |
140 (92) |
64 (42) |
19 (13) |
137 (92) |
52 (35) |
12 (8) |
|
Thrombocytopenia |
127 (84) |
22 (14) |
25 (16) |
118 (79) |
14 (9) |
22 (15) |
SARCLISA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of overall side effects was similar in men and women. The occurrence of infections was higher in men than women.
- Race: The majority of patients were White. Differences in side effects among races could not be determined.
- Age: The occurrence of overall side effect was similar in patients younger and older than 65 years. The occurence of infections was higher in patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize treatment emergent adverse events (TEAEs) and the most common adverse reactions in infections and investations system organ class (SOC) by sex and age subgroups. Subgroups by race were not analyzed because of predominance of White patients.
Table 5. Summary of TEAEs by Subgroups
|
Demographic Parameters |
SARCLISA + Pomalidomide + Dexamethasone n/N (%) |
Pomalidomide + Dexamethasone n/N (%) |
|
Sex |
||
|
Men |
87/88 (98.9) |
66/68 (97.1) |
|
Women |
64/64 (100) |
80/81 (98.8) |
|
Age Group |
||
|
>65 years |
53/54 (98.1) |
66/68 (97.1) |
|
65 years to 75 years |
66/66 (100) |
52/53 (98.1) |
|
≥ 75 years |
32/32 (100) |
28/28 (100) |
Clinical Trial Data
Table 6. Summary of Infections and Infestations SOC by Subgroups
|
Demographic Parameters |
SARCLISA + Pomalidomide + Dexamethasone n/N (%) |
Pomalidomide + Dexamethasone n/N (%) |
|
Sex |
||
|
Men |
76/88 (86.4) |
42/68 (61.8) |
|
Women |
47/64 (73.4) |
54/81 (66.7) |
|
Age Group |
||
|
>65 years |
40 /54 (74.1) |
47/68 (69.1) |
|
65 years to 75 years |
57/66 (86.4) |
30/53 (56.6) |
|
≥ 75 years |
26/32 (81.3) |
19/28 (67.9) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved SARCLISA based on evidence from a clinical trial (NCT02990338) of 307 patients with previously treated multiple myeloma. The trial was conducted at 102 sites in Europe, North America, Asia, Australia and New Zealand.
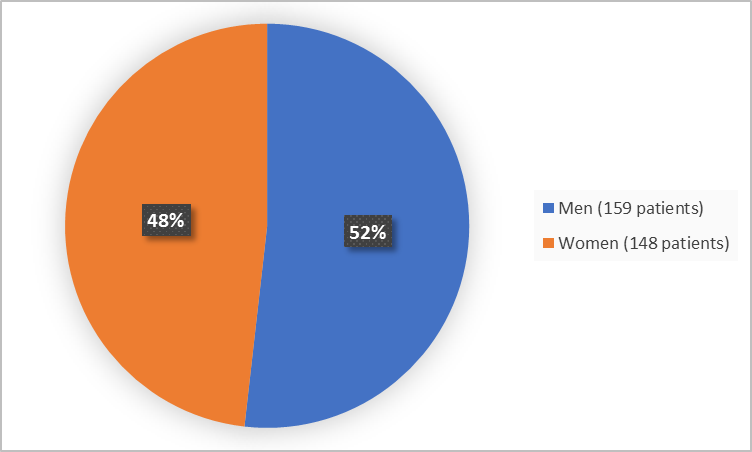
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex (efficacy population)
FDA Review
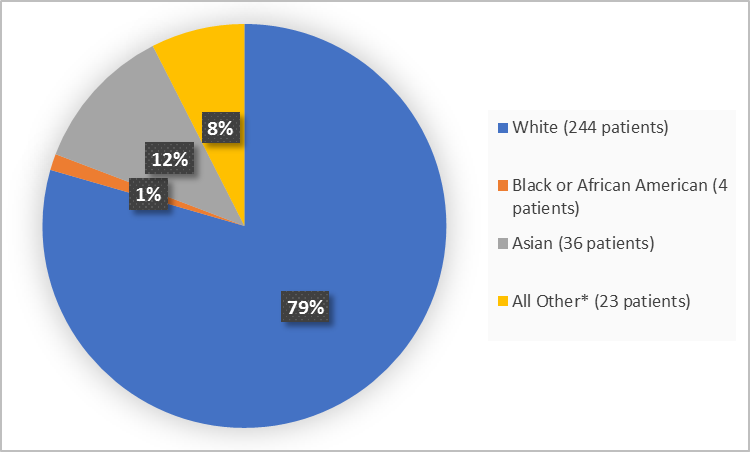
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (efficacy population)
*Includes Native Hawaiian or other Pacific Islander,missing and not reported FDA Review
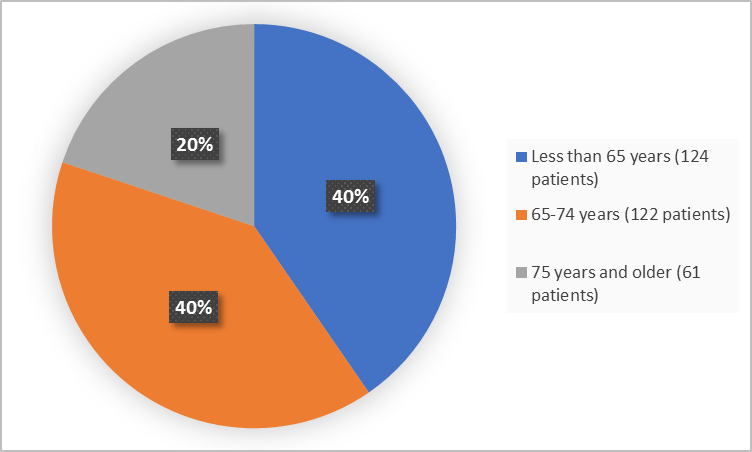
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Baseline Demographics by Age (efficacy population)
FDA Review
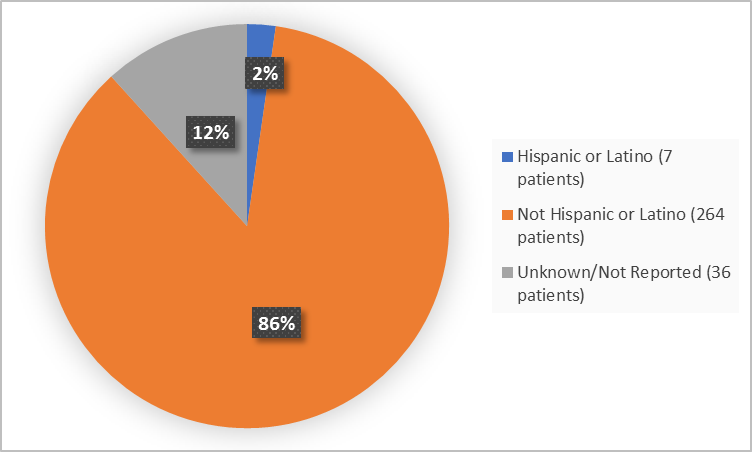
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trial.
Figure 4. Baseline Demographics by Ethnicity (efficacy population)
FDA Review
Who participated in the trials?
The table below summarizes efficacy populations from the trial.
Table 7. Demographic Characteristics (efficacy population)
|
|
SARCLISA + Pomalidomide + Dexamethasone |
Pomalidomide + Dexamethasone |
All |
|
Sex, n (%) |
|
|
|
|
Women |
65 (42.2) |
83 (54.2) |
148 (48.2) |
|
Men |
89 (57.8) |
70 (45.8) |
159 (51.8) |
|
Race, n (%) |
|
|
|
|
White |
118 (76.6) |
126 (82.4) |
244 (79.5) |
|
Black or African American |
1 (0.6) |
3 (2.0) |
4 (1.3) |
|
Asian |
21 (13.6) |
15 (9.8) |
36 (11.7) |
|
Native Hawaiian or other Pacific Island |
2 (1.3) |
1 (0.7) |
3 (1.0) |
|
Missing/Not reported |
12 (7.8) |
8 (5.2) |
20 (6.5) |
|
Age, (years) |
|
|
|
|
Mean (SD) |
66.6 (9.1) |
65.2 (9.5) |
65.9 (9.3) |
|
Median |
68.0 |
66.0 |
67.0 |
|
Min ; Max |
36 ; 83 |
41 ; 86 |
36 ; 86 |
|
Age group, n (%) |
|
|
|
|
<65 years |
54 (35.1) |
70 (45.8) |
124 (40.4) |
|
65-75 years |
68 (44.2) |
54 (35.3) |
122 (39.7) |
|
≥75 years |
32 (20.8) |
29 (19.0) |
61 (19.9) |
|
Ethnicity, n (%) |
|
|
|
|
Hispanic or Latino |
4 (2.6) |
3 (2.0) |
7 (2.3) |
|
Not Hispanic or Latino |
130 (84.4) |
134 (87.6) |
264 (86.0) |
|
Unknown |
2 (1.3) |
2 (1.3) |
4 (1.3) |
|
Not Reported |
18 (11.7) |
14 (9.2) |
32 (10.4) |
|
Geographical Region, n (%) |
|
|
|
|
Western Europe |
55 (35.7) |
76 (49.7) |
131 (42.7) |
|
Eastern Europe |
28 (18.2) |
20 (13.1) |
48 (15.6) |
|
USA |
3 (1.94) |
4 (2.61) |
7 (2.28) |
|
Canada |
4 (2.59) |
1 (0.65) |
5 (1.62) |
|
Asia |
21 (13.6) |
15 (9.8) |
36 (11.7) |
|
Other Countries* |
43 (27.9) |
37 (24.2) |
80 (26.1) |
*Australia, New Zealand, Turkey and Russia
Adapted from FDA Reveiw
How were the trials designed?
There was one trial that evaluated the efficacy and side effects of SARCLISA in patients with previously treated multiple myeloma. Patients were randomly assigned to receive either SARCLISA (in combination with pomalidomide and low-dose dexamethasone) or active comparator (pomalidomide and low-dose dexamethasone).Treatment was administered in both groups in 28-day cycles until disease progression or unacceptable toxicity. Both patients and health care providers knew which treatment was given.
The trial measured the time patients lived without the cancer growing (progression-free survival or PFS).
How were the trials designed?
The efficacy and safety of SARCLISA in combination with pomalidomide and low-dose dexamethasone were evaluated in a multicenter, randomized, open-label trial in patients who had had received at least two prior therapies including lenalidomide and a proteasome inhibitor. Patients were randomized in a 1:1 ratio to receive either SARCLISA in combination with pomalidomide and low-dose dexamethasone or pomalidomide and low-dose dexamethasoneTreatment was administered in both groups in 28-day cycles until disease progression or unacceptable toxicity. The primary endpoint was progression-free survival (PFS). PFS results were assessed by an Independent Response Committee based on central laboratory data for M-protein and central radiologic imaging review using the International Myeloma Working Group (IMWG) criteria.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.