Drug Trials Snapshots: TRIKAFTA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TRIKAFTA Package Insert for complete information.
TRIKAFTA (elexacaftor/tezacaftor/ivacaftor; ivacaftor)
(tri-KAF-tuh)
Vertex Pharmaceuticals
Approval date: October 21, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRIKAFTA is a drug for the treatment of cystic fibrosis (CF) in patients 12 years and older, who have the most common CF mutation (F508del mutation in the cystic fibrosis transmembrane conductance regulator [CFTR] gene).
CF is a rare and serious genetic disorder that results in the formation of thick mucus that builds up in the lungs and other parts of the body. This can lead to severe breathing problems.
How is this drug used?
TRIKAFTA treatment comes in co-packaged orange and blue tablets. Two orange tablets (containing a combination of three drugs elexacaftor, tezacaftor and ivacaftor) are taken by mouth in the morning. One light blue tablet (containing ivacaftor) is taken by mouth, in the evening.
What are the benefits of this drug?
TRIKAFTA improved lung function by allowing air to move easier. In patients treated with TRIKAFTA the amount of air that can be forcibly blown out in one second [percent predicted forced expiratory volume (ppFEV1)] increased.
What are the benefits of this drug (results of trials used to assess efficacy)?
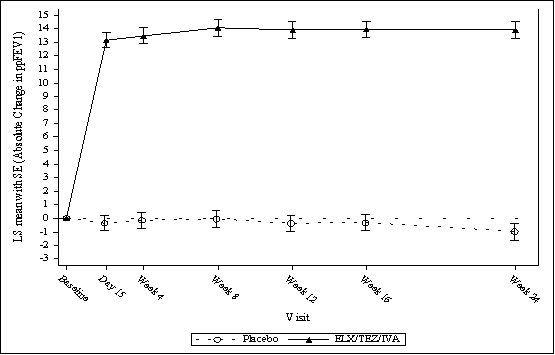
Presented below are the results for Trial 1 and 2. The primary efficacy endpoint was change from baseline in absolute ppFEV1 at Week 4. The absolute change in ppFEV1 through 24 weeks was a key secondary point in Trial 1.
Figure 4. Trial 1- Absolute Change from Baseline in Percent Predicted FEV1 at Each Visit
Table 2. Trial 2-Absolute Change in ppFEV1 from Baseline at Week 4
|
Analysis |
Statistic |
Treatment Difference for TRIKAFTA (N=55) vs Tezacaftor/Ivacaftor# (N=52) |
|
Primary |
||
|
Absolute change in ppFEV1 from baseline at Week 4 (percentage points) |
Treatment difference (95% CI) |
10.0 (7.4, 12.6) |
# Regimen of tezacaftor 100 mg qd/ivacaftor 150 mg q12hr.
TRIKAFTA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TRIKAFTA worked similarly in males and females.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: TRIKAFTA worked similarly in patients younger and older than 18 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
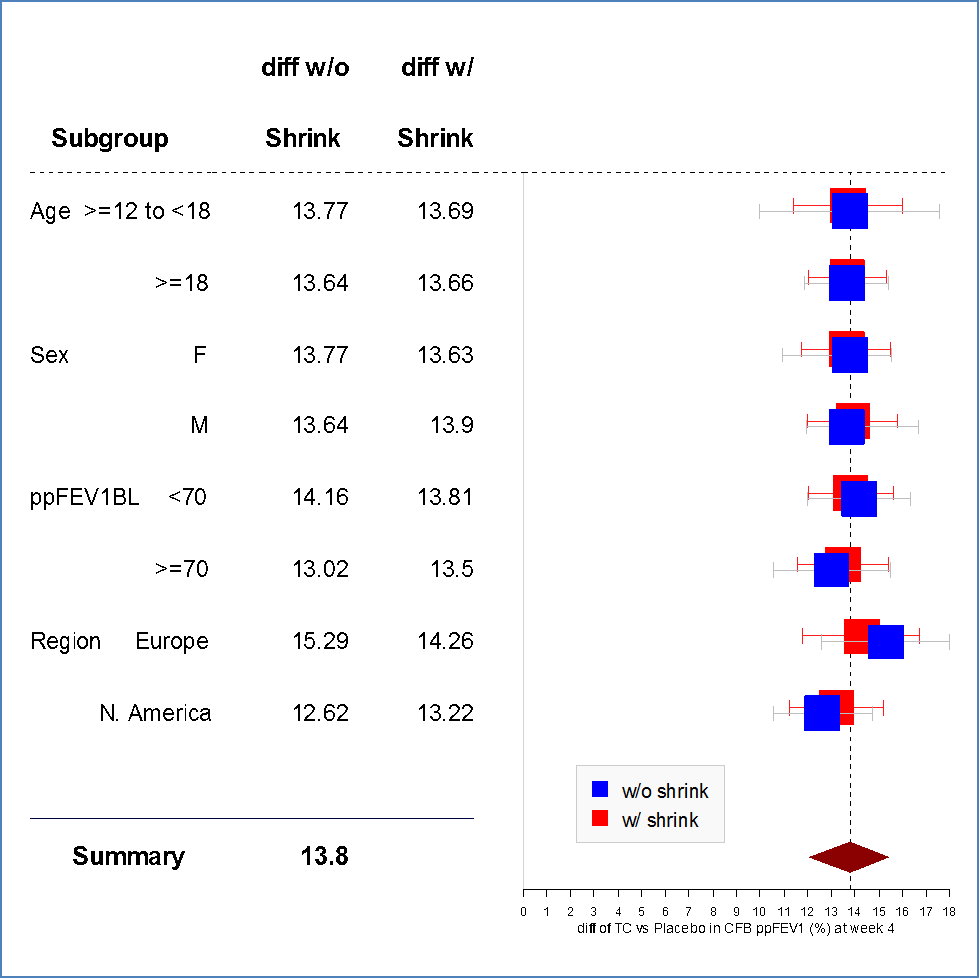
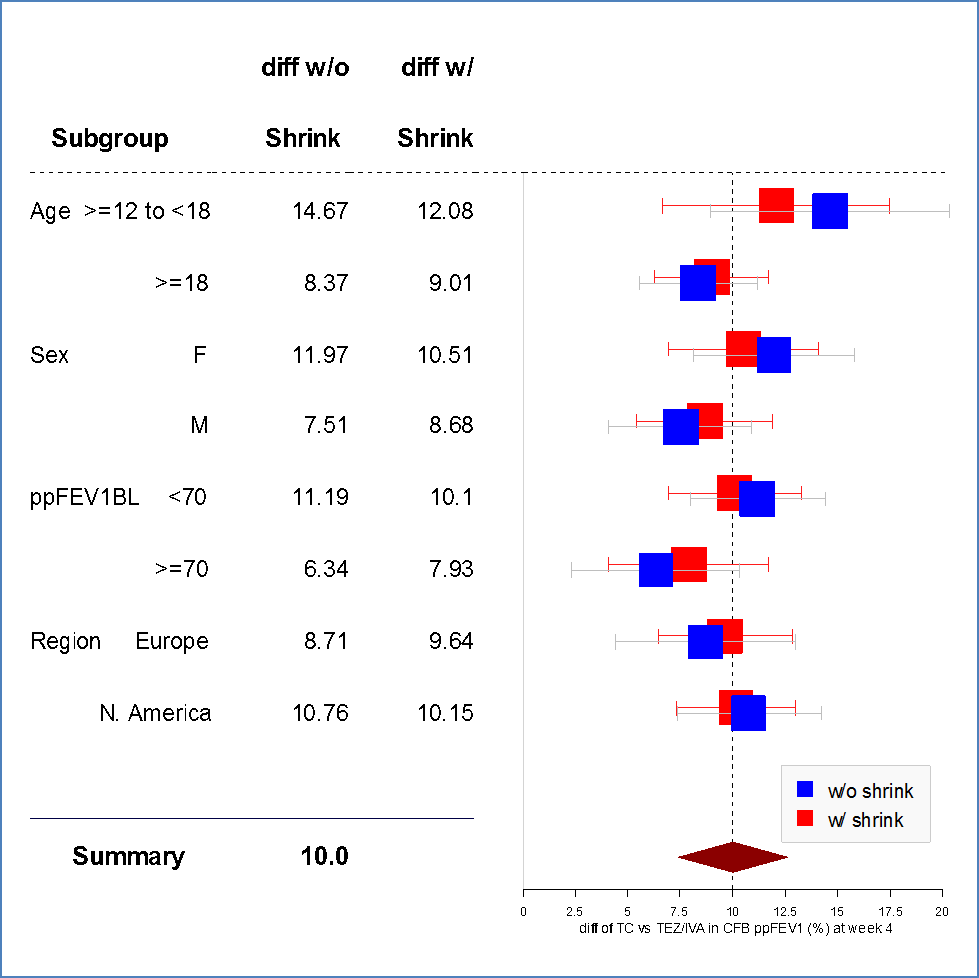
The figures below summarize the primary endpoint, the change in ppFEV1, results by age and sex subgroup for Trials 1 and 2. Race differences were not assessed because of the small number of patients in races other than White.
Figure 4. Least Squares Mean Difference Between Treatments With 95% CI for Change From Baseline in ppFEV1 at Week 4 by Subgroup Using Bayesian Shrinkage Subgroup Analysis (iFAS, Trial 1)
Figure 5. Least Squares Mean Difference Between Treatments With 95% CI for Change From Baseline in ppFEV1 at Week 4 by Subgroup Using Bayesian Shrinkage Subgroup Analysis (FAS, Trial 2)
FDA Review
What are the possible side effects?
TRIKAFTA may cause serious side effects including increased liver enzymes and clouding of the lens in the eye (cataracts).
The most commonly reported side effects associated with TRIKAFTA are headache, upper respiratory infections, abdominal pain, diarrhea, rash and elevated liver enzymes.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes common adverse reactions for safety population from Trial 1.
Table 3. Incidence of Adverse Drug Reactions in ≥5% of TRIKAFTA- Treated Patients and Greater than Placebo by ≥ 1%
|
Adverse Drug Reactions |
TRIKAFTA |
Placebo |
|
Headache |
35 (17) |
30 (15) |
|
Upper respiratory tract infectiona |
32 (16) |
25 (12) |
|
Abdominal painb |
29 (14) |
18 (9) |
|
Diarrhea |
26 (13) |
14 (7) |
|
Rashc |
21 (10) |
10 (5) |
|
Alanine aminotransferase increased |
20 (10) |
7 (3) |
|
Nasal congestion |
19 (9) |
15 (7) |
|
Blood creatine phosphokinase increased |
19 (9) |
9 (4) |
|
Aspartate aminotransferase increased |
19 (9) |
4 (2) |
|
Rhinorrhea |
17 (8) |
6 (3) |
|
Rhinitis |
15 (7) |
11 (5) |
|
Influenza |
14 (7) |
3 (1) |
|
Sinusitis |
11 (5) |
8 (4) |
|
Blood bilirubin increased |
10 (5) |
2 (1) |
a Includes upper respiratory tract infection and viral upper respiratory tract infection
b Includes abdominal pain, abdominal pain upper, abdominal pain lower
c Includes: rash, rash generalized, rash erythematous, rash macular, rash pruritic
TRIKAFTA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The overall occurrence of side effects was similar in males and females, with exception of headache and rash side effects, which occurred more frequently in females.
- Race: Most of the patients in the trials were White. Differences in the occurrence of side effects among races could not be determined, because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients less than and older than 18 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize selected common adverse reactions (headache and rash) by sex and age subgroup. Race differences were not assessed because of the small number of patients in races other than White.
Table 4. Subgroup Analysis of Headache (Trial 1-safety population)
|
Demographic Parameter |
TRIKAFTA |
Placebo |
|
Sex |
|
|
|
Male |
13/104 (13) |
16/105 (15) |
|
Female |
22/98 (22) |
14/96 (15) |
|
Age |
|
|
|
<18 years |
5/56 (9) |
10/60 (17) |
|
≥18 years |
30/146 (20) |
20/141 (14) |
Table 5. Subgroup Analysis of Rash (Trial 1-safety population)
|
Demographic Parameter |
TRIKAFTA |
Placebo |
|
Sex |
|
|
|
Male |
5/104 (5) |
4/105 (4) |
|
Female |
16/98 (16) |
6/96 (6) |
|
Age |
|
|
|
<18 years |
6/56 (11) |
2/60 (3) |
|
≥18 years |
15/146 (10) |
8/141 (6) |
Adapted from FDA Review
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved TRIKAFTA based on evidence from 2 clinical trials (Trial 1/NCT03525444 and Trial 2/NCT03525548) of 510 patients 12-64 years of age with cystic fibrosis and at least one F508del mutation.
Trials were conducted at 154 sites in USA, Canada, Austria, Belgium, Czech Republic, France, Germany, Greece, Italy, Netherlands, Sweden, UK and Australia.
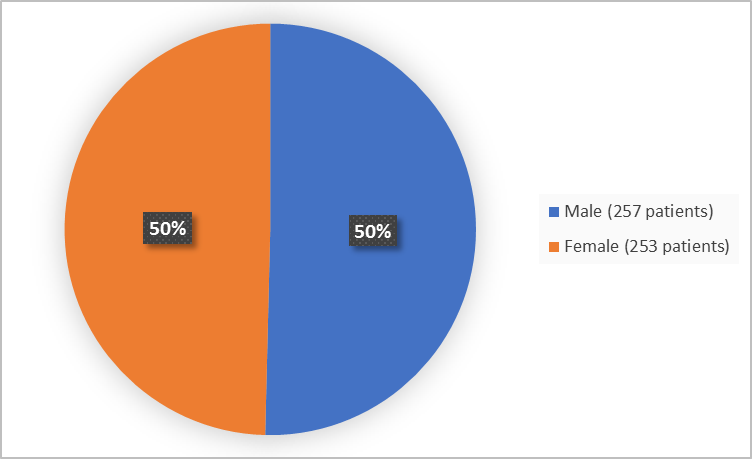
Figures 1 – 3 below summarize by sex, race and age how many patients participated in the combined clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
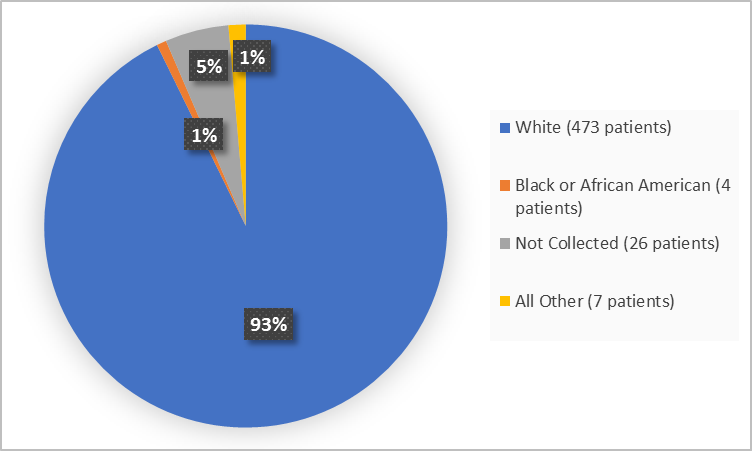
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Efficacy Trials by Race
|
Race |
Number of Patients |
Percentage of Patients |
|
White |
473 |
93 |
|
Black or African American |
4 |
1 |
|
All Other |
7 |
1 |
|
Not Collected* |
26 |
5 |
*Data not collected due to local regulations
FDA Review
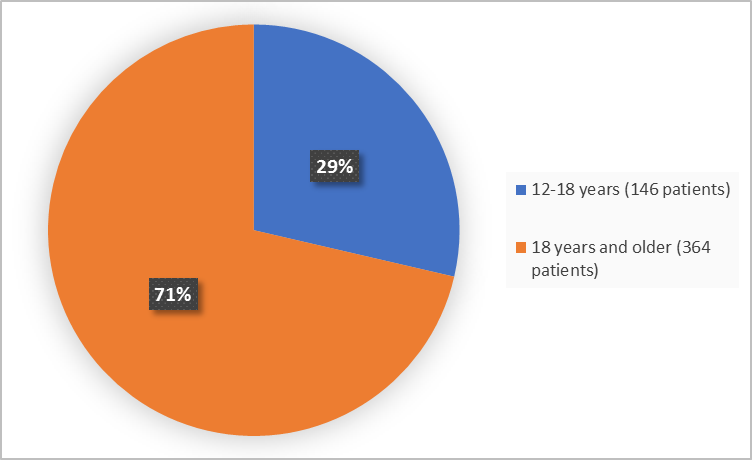
Figure 3. Baseline Demographics by Age
FDA Review
The tables below summarize patients who participated in the trials.
Table 6. Demographic Characteristics of All Participants in Trials 1 and 2
|
Demographic Characteristic |
Trial 1 |
Trial 2 |
TOTAL |
|
Sex |
|
|
|
|
Male |
209 (52) |
48 (45) |
257 (50) |
|
Female |
194 (48) |
59 (55) |
253 (50) |
|
Race |
|
|
|
|
White |
367 (91) |
106 (99) |
473 (93) |
|
Black or African American |
4 (1) |
0 |
4 (1) |
|
American Indian or Alaska Native |
1 (<1) |
0 |
1 (<1) |
|
Multiple |
3 (1) |
0 |
3 (1) |
|
Other |
3 (1) |
0 |
3 (1) |
|
Not collected per local regulations |
25 (6) |
1 (<1) |
26 (5) |
|
Age |
|
|
|
|
Min, max (years) |
12.1, 64.0 |
12.4, 60.5 |
12.1,64 |
|
Age Group |
|
|
|
|
12 to <18 years |
116 (29) |
30 (28) |
146 (29) |
|
18 years to <65 years |
287 (71) |
77 (72) |
364 (71) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
16 (4) |
5 (5) |
21(4) |
|
Not Hispanic or Latino |
362 (90) |
101 (94) |
463 (91) |
|
Not collected per local regulations |
25 (6) |
1 (<1) |
26 (5) |
|
Region |
|
|
|
|
North America |
238 (59) |
67 (63) |
305 (60) |
|
Europe and Australia |
165 (41) |
40 (37) |
205 (40) |
Adapted from FDA Review
Table 7. Demographic Characteristics of Trial 1 (FAS Population)
|
Demographic Characteristic |
TRIKAFTA |
Placebo |
Total |
|
Sex |
|
|
|
|
Male |
104 (52) |
105 (52) |
209 (52) |
|
Female |
96 (48) |
98 (48) |
194 (48) |
|
Race |
|
|
|
|
White |
185 (93) |
182 (90) |
367 (91) |
|
Black or African American |
3 (2) |
1 (<1) |
4 (1) |
|
American Indian or Alaska Native |
0 |
1 (<1) |
1 (<1) |
|
Multiple |
1 (<1) |
2 (1) |
3 (1) |
|
Other |
2 (1) |
1 (<1) |
3 (1) |
|
Not collected per local regulations |
9 (5) |
16 (8) |
25 (6) |
|
Age |
|
|
|
|
Mean years (SD) |
25.6 (9.7) |
26.8 (11.3) |
26.2 (10.5) |
|
Median (years) |
24.4 |
25.0 |
24.4 |
|
Min, max (years) |
12.1, 59.9 |
12.3, 64.0 |
12.1, 64.0 |
|
Age Group |
|
|
|
|
12 to <18 years |
56 (28) |
60 (30) |
116 (29) |
|
18 years to <65 years |
144 (72) |
143 (70) |
287 (71) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
4 (2) |
12 (6) |
16 (4) |
|
Not Hispanic or Latino |
187 (94) |
175 (86) |
362 (90) |
|
Not collected per local regulations |
9 (4) |
16 (8) |
25 (6) |
|
Region |
|
|
|
|
North America |
118 (59) |
120 (59) |
238 (59) |
|
Europe and Australia |
82 (41) |
83 (41) |
165 (41) |
FAS= all randomized subjects who carry the intended CFTR allele mutation and receive at least 1 dose of study drug.
FDA Review
Table 8. Demographic Characteristics of Trial 2 (FAS Population)
|
Demographic Characteristic |
TRIKAFTA |
tezacaftor/ivacaftor |
Total |
|
Sex |
|
|
|
|
Male |
24 (44) |
24 (46) |
48 (45) |
|
Female |
31 (56) |
28 (54) |
59 (55) |
|
Race |
|
|
|
|
White |
54 (98) |
52 (100) |
106 (99) |
|
Black or African American |
0 |
0 |
0 |
|
American Indian or Alaska Native |
0 |
0 |
0 |
|
Multiple |
0 |
0 |
0 |
|
Other |
0 |
0 |
0 |
|
Not collected per local regulations |
1 (2) |
0 |
1 (<1) |
|
Age |
|
|
|
|
Mean years (SD) |
28.8 (11.5) |
27.9 (10.8) |
28.4 (11.1) |
|
Median (years) |
27.4 |
27.6 |
27.4 |
|
Min, max (years) |
12.7, 54.1 |
12.4, 60.5 |
12.4, 60.5 |
|
Age Group |
|
|
|
|
12 to <18 years |
16 (29) |
14 (27) |
30 (28) |
|
18 years to <65 years |
39 (71) |
38 (73) |
77 (72) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
2 (4) |
3 (6) |
5 (5) |
|
Not Hispanic or Latino |
52 (94) |
49 (94) |
101 (94) |
|
Not collected per local regulations |
1 (2) |
0 |
1 (<1) |
|
Region |
|
|
|
|
North America |
34 (62) |
33 (63) |
67 (63) |
|
Europe and Australia |
21 (38) |
19 (37) |
40 (37) |
FAS= all randomized subjects who carry the intended CFTR allele mutation and receive at least 1 dose of study drug.
FDA Review
How were the trials designed?
The benefit and side effects of TRIKAFTA were evaluated in two trials.
Trial 1 was conducted in CF patients, who have one copy of the F508del mutation. Patients were randomly assigned to receive TRIKAFTA, or placebo twice daily for 24 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
Trial 2 was conducted in CF patients who have 2 copies of F508del mutation. Patients were randomly assigned to receive TRIKAFTA, or an active control (an approved CF drug tezacaftor/ivacaftor) twice daily for 4 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
In both trials, the benefit of TRIKAFTA was assessed by change in ppFEV1, the amount of air that can be forcibly blown out in one second. In Trial 1 TRIKAFTA was compared to placebo after 4 weeks of treatment and in Trial 2 to active comparator after 4 weeks of treatment.
How were the trials designed?
There were two randomized, double-blind, multicenter, controlled trials (Trials 1 and 2) that evaluated TRIKAFTA in patients with CF aged 12 years and older.
Trial 1 evaluated TRIKAFTA in patients who are heterozygous for the F508del mutation (F508del mutation on one allele and a mutation on the second allele that results in either no CFTR protein or a CFTR protein that is predicted to be unresponsive to ivacaftor and tezacaftor/ivacaftor therapy). Patients received twice daily TRIKAFTA or placebo for 24 weeks. The primary efficacy endpoint was change in lung function as determined by absolute change from baseline in ppFEV1 at Week 4.
Trial 2 evaluated TRIKAFTA in patients, who are homozygous for the F508del mutation on the CFTR gene. Patients received twice daily TRIKAFTA or tezacaftor/ivacaftor. The primary efficacy endpoint was change in lung function as determined by the mean absolute change from baseline in ppFEV1 at Week 4.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.