Drug Trials Snapshots: UNITUXIN
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the UNITUXIN Prescribing Information for complete information.
UNITUXIN (dinutuximab)
(yoo-ni-TUX-in)
United Therapeutics Corporation
Approval date: Approval date: March 10, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
UNITUXIN is a drug that is used to treat children with high-risk neuroblastoma, a rare and serious cancer that develops from nerve cells and most often occurs in young children. UNITUXIN is used to treat children whose tumor has shrunk following other treatments, which include a combination of chemotherapy, surgery, and radiation.
How is this drug used?
UNITUXIN is given as a slow infusion (taking 10-20 hours) into a vein. Three other drugs, 13-cis-retinoic acid (RA), granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2), are given in a specific order along with UNITUXIN.
What are the benefits of this drug?
In the trial that supported the FDA approval of UNITUXIN combined with IL-2, GM-CSF, and RA, 63% of patients who received the UNITUXIN combination were alive and had no growth or recurrence of their tumor three years after enrollment, compared to 46% of patients who received RA alone.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the trial.
Table 2. Efficacy Results for the Clinical Trial (Intent to Treat Population)
| Efficacy Parameter | UNITUXIN/RA arm N=113 | RA arm N=113 | |
|---|---|---|---|
| EFS | Number of Events (%) | 33 (29) | 50 (44) |
| Median (95% CI) (years) | NR (3.4, NR) | 1.9 (1.3, NR) | |
| Hazard Ratio (95 % CI) | 0.57 (0.37, 0.89) | ||
| p-value (log-rank test)1 | 0.01 | ||
| OS2 | Number of Events | 31 (27) | 48 (42) |
| Median (95% CI) (years) | NR (7.5, NR) | NR (3.9, NR) | |
| Hazard Ratio (95 % CI) | 0.58 (0.37, 0.91) | ||
RA=13-cis-retinoic acid; NR=not reached; EFS= event-free survival; OS= overall survival
1 Compared to the allocated alpha of 0.01 pre-specified for the seventh interim analysis of EFS
2 Based on an additional three years of follow up after the seventh interim analysis of EFS
Source: UNITUXIN Package Insert, Section 14, Table 10
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: UNITUXIN was similarly effective in boys and girls.
- Race: The majority of patients were White. Therefore, differences in response to UNITUXIN could not be determined.
- Age: The majority of patients were 2 to 11 years old. Therefore, differences in response to UNITUXIN among patients younger than 2 and older than 11 years could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Table 3. Subgroup Analysis of Event-Free Survival
| Demographic Subgroup | UNITUXIN/RA | RA | Hazard Ratio (95% CI) |
|---|---|---|---|
| Number of EFS events/n | |||
| Sex | |||
| Male | 21/71 | 28/64 | 0.6 (0.4, 1.1) |
| Female | 12/42 | 22/49 | 0.5 (0.2, 1.0) |
| Dichotomized Age | |||
| 18> | 0/4 | 2/4 | 0 |
| ≥18 months | 33/109 | 48/109 | 0.6 (0.4, 0.9) |
| Age Category | |||
| Infant Toddler (Age | 1/12 | 5/18 | 0.3 (0, 2.5) |
| Child (2 to 11 years) | 32/97 | 44/94 | 0.6 (0.4, 0.9) |
| Adolescent (12 to 17 years) | 0/4 | 1/1 | 0 |
| Race | |||
| White | 26/95 | 39/90 | 0.6 (0.3, 0.9) |
| Black or African American | 2/8 | 5/8 | 0.3 (0.1, 1.4) |
| Asian | 1/2 | 2/4 | 0.5 (0, 6.6) |
| Native Hawaiian or Other Pacific Islander | 0/0 | 1/2 | NA |
| Multiple | 0/1 | 1/2 | 0 |
| Other | 0/1 | 0/0 | NA |
| Unknown | 4/6 | 2/7 | 2.5 (0.5, 14.0) |
Source: From Clinical Review
What are the possible side effects?
The most common side effects of UNITUXIN were:
- severe pain
- fever
- low platelet counts which can lead to bleeding
- low numbers of white blood cells which help fight infection
- reactions occurring during or shortly after the infusion of UNITUXIN, including itching and flushing, hives, swelling of the face and lips, and breathing problems
- low blood pressure
Serious side effects were:
- severe pain and possible nerve damage due to the irritation of nerve cells
- life-threatening reactions related to the infusion of UNITUXIN through a vein, including swelling of the upper airways, difficulty breathing, and low blood pressure, that can occur during or shortly after the infusion is done
- leakage of fluid from blood vessels causing swelling and low blood pressure
- infections
- eye problems that can result in loss of sight
- abnormal blood levels of sodium, potassium, and calcium
- low blood cell counts
What are the possible side effects (result of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial.
Table 4. Percentage of Patients with Adverse Reactions Occurring in at least 10% of the Patients in the Unituxin/RA Group in the Clinical Trial (Safety Population)
| Adverse Reaction | UNITUXIN/RA N=134* (%) | RA N=106 (%) |
|---|---|---|
| Pain | 85 | 16 |
| Pyrexia | 72 | 27 |
| Thrombocytopenia | 66 | 43 |
| Lymphopenia | 62 | 36 |
| Infusion Reactions | 60 | 9 |
| Hypotension | 60 | 3 |
| Hyponatremia | 58 | 12 |
| Increased alanine aminotransferase | 56 | 31 |
| Anemia | 51 | 22 |
| Vomiting | 46 | 19 |
| Diarrhea | 43 | 15 |
| Hypokalemia | 43 | 4 |
| Capillary leak syndrome | 40 | 1 |
| Neutropenia | 39 | 16 |
| Urticaria | 37 | 3 |
| Hypoalbuminemia | 33 | 3 |
| Increased aspartate aminotransferase | 28 | 7 |
| Hypocalcemia | 27 | 0 |
| Hypoxia | 24 | 2 |
| Hypophosphatemia | 20 | 3 |
| Tachycardia | 19 | 1 |
| Hyperglycemia | 18 | 4 |
| Sepsis | 18 | 9 |
| Hemorrhage | 17 | 6 |
| Edema | 17 | 0 |
| Hypertriglyceridemia | 16 | 11 |
| Device related infection | 16 | 11 |
| Proteinuria | 16 | 3 |
| Increased serum creatinine | 15 | 6 |
| Decreased appetite | 15 | 5 |
| Hypertension | 14 | 7 |
| Peripheral neuropathy | 13 | 6 |
| Hypomagnesemia | 12 | 1 |
| Increased weight | 10 | 0 |
| Nausea | 10 | 3 |
* Unituxin safety population includes 109 randomized patients and 25 patients with biopsy-proven residual disease who were non-randomly assigned to receive Unituxin
Source: Extracted from UNITUXIN Package Insert, Section 6, Table 5
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of overall side effects was similar in males and females.
- Race: The majority of patients were White. Therefore, differences in side effects among races could not be determined.
- Age: The majority of patients were 2 to 11 years old. Therefore, differences in side effects among patients younger than 2 and older than 11 years could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The following table summarizes adverse reactions by System Organ Class (Body System) in males and females in the pivotal trial.
Table 5. Subgroup Analysis of Adverse Reactions in Males and Females by Body System (Safety Population)
| Body System | Male N=139 | Female N=101 | ||
|---|---|---|---|---|
| UNITUXIN/RA n=80* n (%) | RA n=59 n (%) | UNITUXIN/RA n=54* n (%) | RA n=47 n (%) | |
| Blood and Lymphatic System Disorders | 62 (78) | 30 (51) | 44 (81) | 24 (51) |
| Cardiac Disorders | 17 (21) | 1 (2) | 10 (19) | 1 (2) |
| Gastrointestinal Disorders | 46 (57) | 21 (36) | 34 (63) | 14 (30) |
| General disorders and Administration Site Conditions | 71 (89) | 20 (34) | 52 (96) | 14 (30) |
| Immune System Disorders | 45 (56) | 5 (8) | 35 (65) | 4 (9) |
| Infections and Infestations | 41 (51) | 24 (41) | 33 (61) | 23 (49) |
| Investigations | 59 (74) | 35 (59) | 47 (87) | 22 (47) |
| Metabolism and Nutrition Disorders | 60 (75) | 14 (24) | 45 (83) | 15 (32) |
| Nervous System Disorders | 19 (24) | 6 (10) | 13 (24) | 6 (13) |
| Renal and Urinary Disorders | 20 (25) | 1 (2) | 17 (31) | 6 (13) |
| Respiratory, Thoracic, and Mediastinal Disorders | 25 (31) | 6 (10) | 27 (50) | 1 (2) |
| Skin and Subcutaneous Tissue Disorders | 37 (46) | 16 (27) | 30 (56) | 8 (17) |
| Vascular Disorders | 54 (68) | 5 (8) | 46 (85) | 4 (9) |
Source: From Company Submission
* Unituxin safety population includes 109 randomized patients and 25 patients with biopsy-proven residual disease who were non-randomly assigned to receive Unituxin
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved UNITUXIN based on a clinical trial of 226 children with high-risk neuroblastoma who already received treatment for their disease and had shrinkage of their tumor.
The trial was conducted at 90 centers in the United States, Canada, and Australia.
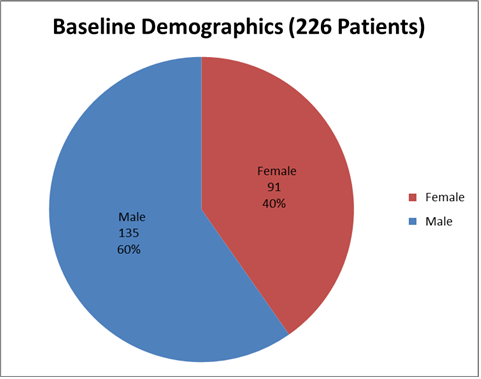
Figure 1 summarizes how many boys and girls were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: From Clinical Reviewer
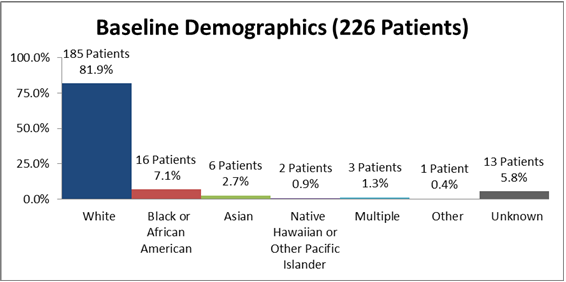
Figure 2 summarizes how many patients by race were in the clinical trial.
Figure 2. Baseline Demographics by Race
Source: From Clinical Reviewer
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage (%) |
|---|---|---|
| White | 185 | 81.9 |
| Black or African American | 16 | 7.1 |
| Asian | 6 | 2.7 |
| Native Hawaiian or Other Pacific Islander | 2 | 0.9 |
| Multiple | 3 | 1.3 |
| Other | 1 | 0.4 |
| Unknown | 13 | 5.8 |
Source: From Clinical Reviewer
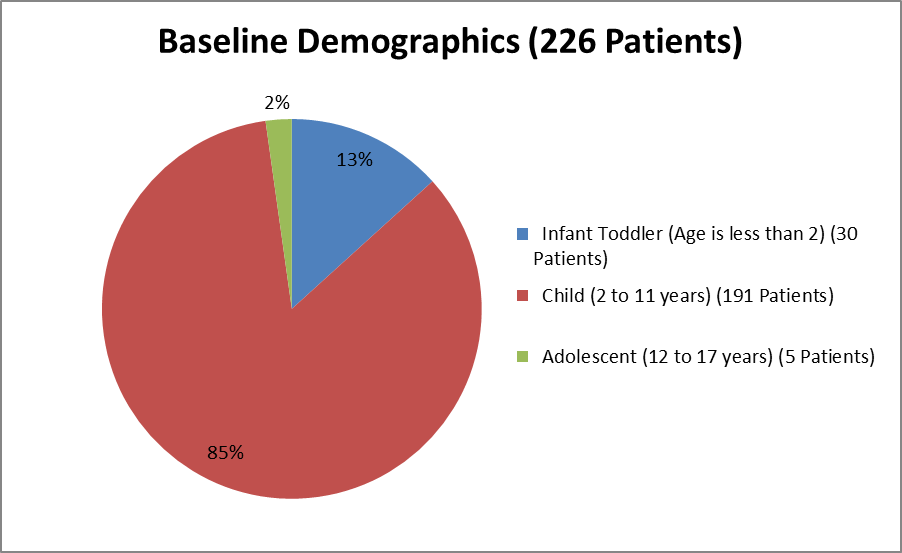
The figure below summarizes the age of patients in the clinical trial.
Figure 3. Baseline Demographics by Age
Source: From Clinical Reviewer
Who participated in the trials?
The table below summarizes baseline demographics for the trial that supported the approval of UNITUXIN.
Table 6. Baseline Demographics (Intent to Treat Population)
| Demographic Parameter | UNITUXIN/RA N=113 | RA N=113 |
|---|---|---|
| Sex n (%) | ||
| Male | 71 (63) | 64 (57) |
| Female | 42 (37) | 49 (43) |
| Age at Enrollment (years) | ||
| Mean (SD) | 4.3 (2.5) | 4.0 (2.1) |
| Median | 3.9 | 3.5 |
| Min, Max | 0.9, 15.3 | 0.9, 13.3 |
| Dichotomized Age n (%) | ||
| 18> | 4 (4) | 4 (4) |
| ≥18 months | 109 (96) | 109 (96) |
| Age Category n (%) | ||
| Infant Toddler (Age | 12 (11) | 18 (16) |
| Child (2 to 11 years) | 97 (86) | 94 (83) |
| Adolescent (12 to 17 years) | 4 (3) | 1 (1) |
| Race n (%) | ||
| White | 95 (84) | 90 (80) |
| Black or African American | 8 (7) | 8 (7) |
| Asian | 2 (2) | 4 (3) |
| Native Hawaiian or Other Pacific Islander | 0 | 2 (2) |
| Multiple | 1 (1) | 2 (2) |
| Other | 1 (1) | 0 |
| Unknown | 6 (5) | 7 (6) |
| Ethnicity n (%) | ||
| Hispanic or Latino | 11 (10) | 11 (10) |
| Not Hispanic or Latino | 100 (88) | 96 (85) |
| Unknown | 2 (2) | 6 (5) |
| Country n (%) | ||
| Australia | 1 (1) | 3 (3) |
| Canada | 11 (10) | 13 (11) |
| United States | 101 (89) | 97 (86) |
SD=standard deviation
Source: From Company Submission
Table 7. Baseline Demographics (Safety Population)
| UNITUXIN/RA N=134 | RA N=106 | |
|---|---|---|
| Demographic Subgroup | ||
| Sex | ||
| Male | 80 (60) | 59 (56) |
| Female | 54 (40) | 47 (44) |
| Age at Enrollment (years) | ||
| N | 134 | 106 |
| Mean (SD) | 4.6 (3) | 4 (2) |
| Median | 4 | 3.6 |
| Min, Max | 1.0, 15.3 | 1.0, 13.3 |
| Dichotomized Age | ||
| 18> | 3 (2) | 3 (3) |
| ≥18 months | 131 (98) | 103 (97) |
| Age Category n (%) | ||
| Infant toddler (age | 12 (9) | 17 (16) |
| Child (2 to 11 years) | 116 (87) | 88 (83) |
| Adolescent (12 to 17 years) | 6 (5) | 1 (1) |
| Race n (%) | ||
| White | 108 (81) | 84 (79) |
| Black or African American | 12 (9) | 7 (6.6) |
| Asian | 4 (3) | 4 (3.8) |
| Native Hawaiian or Other Pacific Islander | 0 | 2 (2) |
| American Indian or Alaska Native | 0 | 0 |
| Multiple | 2 (2) | 2 (2) |
| Other | 1 (0.7) | 0 |
| Unknown | 7 (5.2) | 7 (6.6) |
| Ethnicity n (%) | ||
| Hispanic or Latino | 15 (11) | 10 (9) |
| Not Hispanic or Latino | 117 (87) | 90 (85) |
| Unknown | 2 (1.5) | 6 (5.7) |
Source: From Company Submission
How were the trials designed?
The trial randomly assigned half of the 226 patients enrolled to receive standard treatment (13-cis-retinoic acid, or RA) for 6 treatment cycles and the other half to receive UNITUXIN in combination with RA and GM-CSF (during Cycles 1, 3, and 5) and in combination with RA and IL-2 (during Cycles 2 and 4) followed by one cycle of RA alone.
The trial compared the amount of time from trial enrollment in the two treatment groups to either 1) growth of the neuroblastoma tumor or 2) patient death.
How were the trials designed?
The safety and effectiveness of Unituxin were evaluated in a randomized, open-label, multicenter trial conducted in pediatric patients with high-risk neuroblastoma. All patients had received prior therapy consisting of induction combination chemotherapy, maximum feasible surgical resection, myeloablative consolidation chemotherapy followed by autologous stem cell transplant, and radiation therapy to residual soft tissue disease. Patients were randomized between Day 50 and Day 77 post-autologous stem cell transplantation.
The major efficacy outcome measure was investigator-assessed event-free survival (EFS), defined as the time from randomization to the first occurrence of relapse, progressive disease, secondary malignancy, or death. Overall survival (OS) was also evaluated. After observing a numerical improvement in EFS based on the seventh interim analysis, the Data Monitoring Committee recommended termination of accrual.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
MEDICAL REVIEW